Persistence of coordinated long-term potentiation and dendritic spine enlargement at mature hippocampal CA1 synapses requires N-cadherin
- PMID: 20668183
- PMCID: PMC2921177
- DOI: 10.1523/JNEUROSCI.1223-10.2010
Persistence of coordinated long-term potentiation and dendritic spine enlargement at mature hippocampal CA1 synapses requires N-cadherin
Abstract
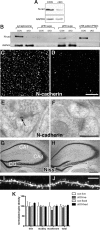
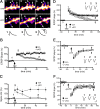
Persistent changes in spine shape are coupled to long-lasting synaptic plasticity in hippocampus. The molecules that coordinate such persistent structural and functional plasticity are unknown. Here, we generated mice in which the cell adhesion molecule N-cadherin was conditionally ablated from postnatal, excitatory synapses in hippocampus. We applied to adult mice of either sex a combination of whole-cell recording, two-photon microscopy, and spine morphometric analysis to show that postnatal ablation of N-cadherin has profound effects on the stability of coordinated spine enlargement and long-term potentiation (LTP) at mature CA1 synapses, with no effects on baseline spine density or morphology, baseline properties of synaptic neurotransmission, or long-term depression. Thus, N-cadherin couples persistent spine structural modifications with long-lasting synaptic functional modifications associated selectively with LTP, revealing unexpectedly distinct roles at mature synapses in comparison with earlier, broader functions in synapse and spine development.
Figures
References
-
- Bozdagi O, Shan W, Tanaka H, Benson DL, Huntley GW. Increasing numbers of synaptic puncta during late-phase LTP: N-cadherin is synthesized, recruited to synaptic sites, and required for potentiation. Neuron. 2000;28:245–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
