SIRT1 promotes the central adaptive response to diet restriction through activation of the dorsomedial and lateral nuclei of the hypothalamus
- PMID: 20668205
- PMCID: PMC2922851
- DOI: 10.1523/JNEUROSCI.1385-10.2010
SIRT1 promotes the central adaptive response to diet restriction through activation of the dorsomedial and lateral nuclei of the hypothalamus
Abstract
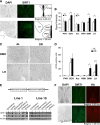
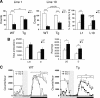
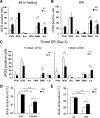
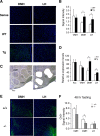
Diet restriction retards aging and extends lifespan by triggering adaptive mechanisms that alter behavioral, physiological, and biochemical responses in mammals. Little is known about the molecular pathways evoking the corresponding central response. One factor that mediates the effects of diet restriction is the mammalian nicotinamide adenine dinucleotide (NAD)-dependent deacetylase SIRT1. Here we demonstrate that diet restriction significantly increases SIRT1 protein levels and induces neural activation in the dorsomedial and lateral hypothalamic nuclei. Increasing SIRT1 in the brain of transgenic (BRASTO) mice enhances neural activity specifically in these hypothalamic nuclei, maintains a higher range of body temperature, and promotes physical activity in response to different diet-restricting paradigms. These responses are all abrogated in Sirt1-deficient mice. SIRT1 upregulates expression of the orexin type 2 receptor specifically in these hypothalamic nuclei in response to diet-restricting conditions, augmenting response to ghrelin, a gut hormone whose levels increase in these conditions. Our results suggest that in the hypothalamus, SIRT1 functions as a key mediator of the central response to low nutritional availability, providing insight into the role of the hypothalamus in the regulation of metabolism and aging in mammals.
Figures


References
-
- Aton SJ, Block GD, Tei H, Yamazaki S, Herzog ED. Plasticity of circadian behavior and the suprachiasmatic nucleus following exposure to non-24-hour light cycles. J Biol Rhythms. 2004;19:198–207. - PubMed
-
- Berkefeld H, Sailer CA, Bildl W, Rohde V, Thumfart JO, Eble S, Klugbauer N, Reisinger E, Bischofberger J, Oliver D, Knaus HG, Schulte U, Fakler B. BKCa-Cav channel complexes mediate rapid and localized Ca2+-activated K+ signaling. Science. 2006;314:615–620. - PubMed
-
- Bishop NA, Guarente L. Genetic links between diet and lifespan: shared mechanisms from yeast to humans. Nat Rev Genet. 2007a;8:835–844. - PubMed
-
- Bishop NA, Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature. 2007b;447:545–549. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
