Fast sodium channel gating supports localized and efficient axonal action potential initiation
- PMID: 20668206
- PMCID: PMC6633381
- DOI: 10.1523/JNEUROSCI.6335-09.2010
Fast sodium channel gating supports localized and efficient axonal action potential initiation
Abstract
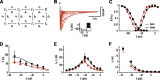
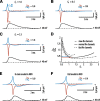
Action potentials (APs) are initiated in the proximal axon of most neurons. In myelinated axons, a 50-times higher sodium channel density in the initial segment compared to the soma may account for this phenomenon. However, little is known about sodium channel density and gating in proximal unmyelinated axons. To study the mechanisms underlying AP initiation in unmyelinated hippocampal mossy fibers of adult mice, we recorded sodium currents in axonal and somatic membrane patches. We demonstrate that sodium channel density in the proximal axon is approximately 5 times higher than in the soma. Furthermore, sodium channel activation and inactivation are approximately 2 times faster. Modeling revealed that the fast activation localized the initiation site to the proximal axon even upon strong synaptic stimulation, while fast inactivation contributed to energy-efficient membrane charging during APs. Thus, sodium channel gating and density in unmyelinated mossy fiber axons appear to be specialized for robust AP initiation and propagation with minimal current flow.
Figures






Similar articles
-
Action potential initiation and propagation in hippocampal mossy fibre axons.J Physiol. 2008 Apr 1;586(7):1849-57. doi: 10.1113/jphysiol.2007.150151. Epub 2008 Feb 7. J Physiol. 2008. PMID: 18258662 Free PMC article.
-
Action potential generation requires a high sodium channel density in the axon initial segment.Nat Neurosci. 2008 Feb;11(2):178-86. doi: 10.1038/nn2040. Epub 2008 Jan 20. Nat Neurosci. 2008. PMID: 18204443
-
High threshold, proximal initiation, and slow conduction velocity of action potentials in dentate granule neuron mossy fibers.J Neurophysiol. 2008 Jul;100(1):281-91. doi: 10.1152/jn.90295.2008. Epub 2008 May 14. J Neurophysiol. 2008. PMID: 18480368 Free PMC article.
-
Neuronal signaling in central nervous system.Sheng Li Xue Bao. 2011 Feb 25;63(1):1-8. Sheng Li Xue Bao. 2011. PMID: 21340428 Review.
-
Excitability tuning of axons in the central nervous system.J Physiol Sci. 2016 May;66(3):189-96. doi: 10.1007/s12576-015-0415-2. Epub 2015 Oct 22. J Physiol Sci. 2016. PMID: 26493201 Free PMC article. Review.
Cited by
-
Input-dependent subcellular localization of spike initiation between soma and axon at cortical pyramidal neurons.Mol Brain. 2014 Apr 4;7:26. doi: 10.1186/1756-6606-7-26. Mol Brain. 2014. PMID: 24708847 Free PMC article.
-
Voltage-Gated Sodium Channels in Neocortical Pyramidal Neurons Display Cole-Moore Activation Kinetics.Front Cell Neurosci. 2018 Jun 26;12:187. doi: 10.3389/fncel.2018.00187. eCollection 2018. Front Cell Neurosci. 2018. PMID: 29997481 Free PMC article.
-
The energy cost of action potential propagation in dopamine neurons: clues to susceptibility in Parkinson's disease.Front Comput Neurosci. 2013 Mar 18;7:13. doi: 10.3389/fncom.2013.00013. eCollection 2013. Front Comput Neurosci. 2013. PMID: 23515615 Free PMC article.
-
Functional connectivity of intercalated nucleus with medial amygdala: A circuit relevant for chemosignal processing.IBRO Neurosci Rep. 2022 Feb 2;12:170-181. doi: 10.1016/j.ibneur.2022.01.005. eCollection 2022 Jun. IBRO Neurosci Rep. 2022. PMID: 35199098 Free PMC article.
-
Population and individual firing behaviors in sparsely synchronized rhythms in the hippocampal dentate gyrus.Cogn Neurodyn. 2022 Jun;16(3):643-665. doi: 10.1007/s11571-021-09728-4. Epub 2021 Oct 23. Cogn Neurodyn. 2022. PMID: 35603046 Free PMC article.
References
-
- Alle H, Roth A, Geiger JRP. Energy-efficient action potentials in hippocampal mossy fibers. Science. 2009;325:1405–1408. - PubMed
-
- Attwell D, Gibb A. Neuroenergetics and the kinetic design of excitatory synapses. Nat Rev Neurosci. 2005;6:841–849. - PubMed
-
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
