Endothelium-derived but not platelet-derived protein disulfide isomerase is required for thrombus formation in vivo
- PMID: 20668226
- PMCID: PMC2996122
- DOI: 10.1182/blood-2010-04-278184
Endothelium-derived but not platelet-derived protein disulfide isomerase is required for thrombus formation in vivo
Abstract
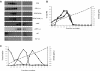
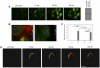
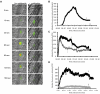
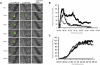
Protein disulfide isomerase (PDI) catalyzes the oxidation reduction and isomerization of disulfide bonds. We have previously identified an important role for extracellular PDI during thrombus formation in vivo. Here, we show that endothelial cells are a critical cellular source of secreted PDI, important for fibrin generation and platelet accumulation in vivo. Functional PDI is rapidly secreted from human umbilical vein endothelial cells in culture upon activation with thrombin or after laser-induced stimulation. PDI is localized in different cellular compartments in activated and quiescent endothelial cells, and is redistributed to the plasma membrane after cell activation. In vivo studies using intravital microscopy show that PDI appears rapidly after laser-induced vessel wall injury, before the appearance of the platelet thrombus. If platelet thrombus formation is inhibited by the infusion of eptifibatide into the circulation, PDI is detected after vessel wall injury, and fibrin deposition is normal. Treatment of mice with a function blocking anti-PDI antibody completely inhibits fibrin generation in eptifibatide-treated mice. These results indicate that, although both platelets and endothelial cells secrete PDI after laser-induced injury, PDI from endothelial cells is required for fibrin generation in vivo.
Figures


References
-
- Chen VM, Hogg PJ. Allosteric disulfide bonds in thrombosis and thrombolysis. J Thromb Haemost. 2006;4:2533–2541. - PubMed
-
- Chen C, Lin Y, Detwiler DC. Protein disulfide isomerase activity is released by activated platelets. Blood. 1992;79(9):2226–2228. - PubMed
-
- Essex DW, Chen K, Swiatkowska M. Localization of protein disulfide isomerase to the external surface of the platelet plasma membrane. Blood. 1995;86(6):2168–2173. - PubMed
-
- Burgess JK, Hotchkiss KA, Suter C, et al. Physical proximity and functional association of glyco-protein-1b alpha and protein disulfide isomerase on the platelet plasma membrane. J Biol Chem. 2000;275(13):9758–9766. - PubMed
-
- Hotchkiss KA, Matthias LJ, Hogg PJ. Exposure of the cryptic Arg-Gly-Asp sequence in thrombospondin-1 by protein disulfide isomerase. Biochim Biophys Acta. 1998;1388(2):478–488. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

