Improved cellular and humoral immune responses in vivo following targeting of HIV Gag to dendritic cells within human anti-human DEC205 monoclonal antibody
- PMID: 20668230
- PMCID: PMC2981538
- DOI: 10.1182/blood-2010-06-288068
Improved cellular and humoral immune responses in vivo following targeting of HIV Gag to dendritic cells within human anti-human DEC205 monoclonal antibody
Abstract
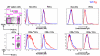
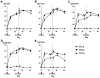
Protein vaccines for T-cell immunity are not being prioritized because of poor immunogenicity. To overcome this hurdle, proteins are being targeted to maturing dendritic cells (DCs) within monoclonal antibodies (mAbs) to DC receptors. To extend the concept to humans, we immunized human immunoglobulin-expressing mice with human DEC205 (hDEC205) extracellular domain. 3D6 and 3G9 mAbs were selected for high-affinity binding to hDEC205. In addition, CD11c promoter hDEC205 transgenic mice were generated, and 3G9 was selectively targeted to DCs in these animals. When mAb heavy chain was engineered to express HIV Gag p24, the fusion mAb induced interferon-γ- and interleukin-2-producing CD4(+) T cells in hDEC205 transgenic mice, if polynocinic polycytidylic acid was coadministered as an adjuvant. The T-cell response was broad, recognizing at least 3 Gag peptides, and high titers of anti-human immunoglobulin G antibody were made. Anti-hDEC205 also improved the cross-presentation of Gag to primed CD8(+) T cells from HIV-infected individuals. In all tests, 3D6 and 3G9 targeting greatly enhanced immunization relative to nonbinding control mAb. These results provide preclinical evidence that in vivo hDEC205 targeting increases the efficiency with which proteins elicit specific immunity, setting the stage for proof-of-concept studies of these new protein vaccines in human subjects.
Figures





References
-
- Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196(12):1627–1638. - PMC - PubMed
-
- Caminschi I, Lahoud MH, Shortman K. Enhancing immune responses by targeting antigen to DC. Eur J Immunol. 2009;39(4):931–938. - PubMed
-
- Boscardin SB, Trumpfheller C, Nussenzweig MC, Steinman RM. Vaccines based on dendritic cell biology. In: Levine MM, editor. New Generation Vaccines. New York, NY: Informa Healthcare; 2009. pp. 327–339. Vol Chapter 32.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

