Quantitative proteomics of the Cav2 channel nano-environments in the mammalian brain
- PMID: 20668236
- PMCID: PMC2930569
- DOI: 10.1073/pnas.1005940107
Quantitative proteomics of the Cav2 channel nano-environments in the mammalian brain
Abstract
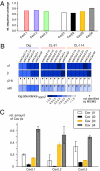
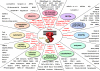
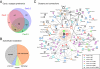
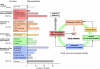
Local Ca(2+) signaling occurring within nanometers of voltage-gated Ca(2+) (Cav) channels is crucial for CNS function, yet the molecular composition of Cav channel nano-environments is largely unresolved. Here, we used a proteomic strategy combining knockout-controlled multiepitope affinity purifications with high-resolution quantitative MS for comprehensive analysis of the molecular nano-environments of the Cav2 channel family in the whole rodent brain. The analysis shows that Cav2 channels, composed of pore-forming alpha1 and auxiliary beta subunits, are embedded into protein networks that may be assembled from a pool of approximately 200 proteins with distinct abundance, stability of assembly, and preference for the three Cav2 subtypes. The majority of these proteins have not previously been linked to Cav channels; about two-thirds are dedicated to the control of intracellular Ca(2+) concentration, including G protein-coupled receptor-mediated signaling, to activity-dependent cytoskeleton remodeling or Ca(2+)-dependent effector systems that comprise a high portion of the priming and release machinery of synaptic vesicles. The identified protein networks reflect the cellular processes that can be initiated by Cav2 channel activity and define the molecular framework for organization and operation of local Ca(2+) signaling by Cav2 channels in the brain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
Age of quantitative proteomics hits voltage-gated calcium channels.Proc Natl Acad Sci U S A. 2010 Aug 24;107(34):14941-2. doi: 10.1073/pnas.1009102107. Epub 2010 Aug 12. Proc Natl Acad Sci U S A. 2010. PMID: 20705901 Free PMC article. No abstract available.
References
-
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. - PubMed
-
- Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. - PubMed
-
- Augustine GJ, Santamaria F, Tanaka K. Local calcium signaling in neurons. Neuron. 2003;40:331–346. - PubMed
-
- Neher E. Vesicle pools and Ca2+ microdomains: New tools for understanding their roles in neurotransmitter release. Neuron. 1998;20:389–399. - PubMed
-
- Fakler B, Adelman JP. Control of K(Ca) channels by calcium nano/microdomains. Neuron. 2008;59:873–881. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

