Second harmonic generating (SHG) nanoprobes for in vivo imaging
- PMID: 20668245
- PMCID: PMC2930484
- DOI: 10.1073/pnas.1004748107
Second harmonic generating (SHG) nanoprobes for in vivo imaging
Abstract
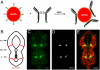
Fluorescence microscopy has profoundly changed cell and molecular biology studies by permitting tagged gene products to be followed as they function and interact. The ability of a fluorescent dye to absorb and emit light of different wavelengths allows it to generate startling contrast that, in the best cases, can permit single molecule detection and tracking. However, in many experimental settings, fluorescent probes fall short of their potential due to dye bleaching, dye signal saturation, and tissue autofluorescence. Here, we demonstrate that second harmonic generating (SHG) nanoprobes can be used for in vivo imaging, circumventing many of the limitations of classical fluorescence probes. Under intense illumination, such as at the focus of a laser-scanning microscope, these SHG nanocrystals convert two photons into one photon of half the wavelength; thus, when imaged by conventional two-photon microscopy, SHG nanoprobes appear to generate a signal with an inverse Stokes shift like a fluorescent dye, but with a narrower emission. Unlike commonly used fluorescent probes, SHG nanoprobes neither bleach nor blink, and the signal they generate does not saturate with increasing illumination intensity. The resulting contrast and detectability of SHG nanoprobes provide unique advantages for molecular imaging of living cells and tissues.
Conflict of interest statement
Conflict of interest statement: This work is the subject of patent applications filed by the California Institute of Technology.
Figures


Comment in
-
Microscope harmonies.Nat Methods. 2010 Oct;7(10):779. doi: 10.1038/nmeth1010-779. Nat Methods. 2010. PMID: 20936770 No abstract available.
References
-
- Prasher DC, Eckenrode VK, Ward WW, Prendergast FG, Cormier MJ. Primary structure of the Aequorea victoria green-fluorescent protein. Gene. 1992;111(2):229–233. - PubMed
-
- Shaner NC, Steinbach PA, Tsien RY. A guide to choosing fluorescent proteins. Nat Methods. 2005;2(12):905–909. - PubMed
-
- Lippincott-Schwartz J, Snapp E, Kenworthy A. Studying protein dynamics in living cells. Nat Rev Mol Cell Biol. 2001;2(6):444–456. - PubMed
-
- Hadjantonakis AK, Dickinson ME, Fraser SE, Papaioannou VE. Technicolour transgenics: Imaging tools for functional genomics in the mouse. Nat Rev Genet. 2003;4(8):613–625. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

