Juvenile ALS with basophilic inclusions is a FUS proteinopathy with FUS mutations
- PMID: 20668261
- PMCID: PMC2931770
- DOI: 10.1212/WNL.0b013e3181ed9cde
Juvenile ALS with basophilic inclusions is a FUS proteinopathy with FUS mutations
Abstract
Background: Juvenile amyotrophic lateral sclerosis (ALS) with basophilic inclusions is a form of ALS characterized by protein deposits in motor neurons that are morphologically and tinctorially distinct from those of classic sporadic ALS. The nosologic position of this type of ALS in the molecular pathologic and genetic classification of ALS is unknown.
Methods: We identified neuropathologically 4 patients with juvenile ALS with basophilic inclusions and tested the hypothesis that specific RNA binding protein pathology may define this type of ALS. Immunohistochemical findings prompted us to sequence the fused in sarcoma (FUS) gene.
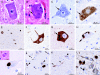
Results: Motor symptoms began between ages 17 and 22. Disease progression was rapid without dementia. No family history was identified. Basophilic inclusions were strongly positive for FUS protein but negative for TAR DNA binding protein 43 (TDP-43). Granular and compact FUS deposits were identified in glia and neuronal cytoplasm and nuclei. Ultrastructure of aggregates was in keeping with origin from fragmented rough endoplasmic reticulum. Sequencing of all 15 exons of the FUS gene in 3 patients revealed a novel deletion mutation (c.1554_1557delACAG) in 1 individual and the c.1574C>T (P525L) mutation in 2 others.
Conclusion: Juvenile ALS with basophilic inclusions is a FUS proteinopathy and should be classified as ALS-FUS. The FUS c.1574C>T (P525L) and c.1554_1557delACAG mutations are associated with this distinct phenotype. The molecular genetic relationship with frontotemporal lobar degeneration with FUS pathology remains to be clarified.
Figures



Comment in
-
FUS mutations in sporadic juvenile ALS: another step toward understanding ALS pathogenesis.Neurology. 2010 Aug 17;75(7):584-5. doi: 10.1212/WNL.0b013e3181ed9ee4. Epub 2010 Jul 28. Neurology. 2010. PMID: 20668260 No abstract available.
References
-
- O'Toole O, Traynor BJ, Brennan P, et al. Epidemiology and clinical features of amyotrophic lateral sclerosis in Ireland between 1995 and 2004. J Neurol Neurosurg Psychiatry 2008;79:30–32. - PubMed
-
- Simpson CL, Al-Chalabi A. Amyotrophic lateral sclerosis as a complex genetic disease. Biochim Biophys Acta 2006;1762:973–985. - PubMed
-
- Rosen DR, Siddique T, Patterson D, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 1993;362:59–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
