Bnip3-mediated mitochondrial autophagy is independent of the mitochondrial permeability transition pore
- PMID: 20668412
- PMCID: PMC3039735
- DOI: 10.4161/auto.6.7.13005
Bnip3-mediated mitochondrial autophagy is independent of the mitochondrial permeability transition pore
Abstract
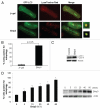
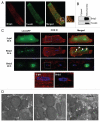
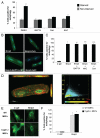
Bnip3 is a pro-apoptotic BH3-only protein which is associated with mitochondrial dysfunction and cell death. Bnip3 is also a potent inducer of autophagy in many cells. In this study, we have investigated the mechanism by which Bnip3 induces autophagy in adult cardiac myocytes. Overexpression of Bnip3 induced extensive autophagy in adult cardiac myocytes. Fluorescent microscopy studies and ultrastructural analysis revealed selective degradation of mitochondria by autophagy in myocytes overexpressing Bnip3. Oxidative stress and increased levels of intracellular Ca(2+) have been reported by others to induce autophagy, but Bnip3-induced autophagy was not abolished by antioxidant treatment or the Ca(2+) chelator BAPT A-AM. We also investigated the role of the mitochondrial permeability transition pore (mPTP) in Bnip3-induced autophagy. Although the mPTP has previously been implicated in the induction of autophagy and selective removal of damaged mitochondria by autophagosomes, mitochondria sequestered by autophagosomes in Bnip3-treated cardiac myocytes had not undergone permeability transition and treatment with the mPTP inhibitor cyclosporine A did not inhibit mitochondrial autophagy in cardiac myocytes. Moreover, cyclophilin D (cypD) is an essential component of the mPTP and Bnip3 induced autophagy to the same extent in embryonic fibroblasts isolated from wild-type and cypD-deficient mice. These results support a model where Bnip3 induces selective removal of the mitochondria in cardiac myocytes and that Bnip3 triggers induction of autophagy independent of Ca(2+), ROS generation, and mPTP opening.
Figures
Similar articles
-
Bnip3 mediates permeabilization of mitochondria and release of cytochrome c via a novel mechanism.J Mol Cell Cardiol. 2010 Jun;48(6):1146-56. doi: 10.1016/j.yjmcc.2009.12.004. Epub 2009 Dec 16. J Mol Cell Cardiol. 2010. PMID: 20025887 Free PMC article.
-
Mitochondrial autophagy by Bnip3 involves Drp1-mediated mitochondrial fission and recruitment of Parkin in cardiac myocytes.Am J Physiol Heart Circ Physiol. 2011 Nov;301(5):H1924-31. doi: 10.1152/ajpheart.00368.2011. Epub 2011 Sep 2. Am J Physiol Heart Circ Physiol. 2011. PMID: 21890690 Free PMC article.
-
Enhancing lysosome biogenesis attenuates BNIP3-induced cardiomyocyte death.Autophagy. 2012 Mar;8(3):297-309. doi: 10.4161/auto.18658. Epub 2012 Feb 3. Autophagy. 2012. PMID: 22302006 Free PMC article.
-
Bnip3 as a dual regulator of mitochondrial turnover and cell death in the myocardium.Pediatr Cardiol. 2011 Mar;32(3):267-74. doi: 10.1007/s00246-010-9876-5. Epub 2011 Jan 6. Pediatr Cardiol. 2011. PMID: 21210091 Free PMC article. Review.
-
Cyclophilin D-mediated Mitochondrial Permeability Transition Regulates Mitochondrial Function.Curr Pharm Des. 2023;29(8):620-629. doi: 10.2174/1381612829666230313111314. Curr Pharm Des. 2023. PMID: 36915987 Review.
Cited by
-
Understanding Anthracycline Cardiotoxicity From Mitochondrial Aspect.Front Pharmacol. 2022 Feb 8;13:811406. doi: 10.3389/fphar.2022.811406. eCollection 2022. Front Pharmacol. 2022. PMID: 35211017 Free PMC article. Review.
-
Mitochondrial Regulation of Diabetic Kidney Disease.Front Med (Lausanne). 2021 Sep 27;8:745279. doi: 10.3389/fmed.2021.745279. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34646847 Free PMC article. Review.
-
Molecular basis of cardioprotective effect of antioxidant vitamins in myocardial infarction.Biomed Res Int. 2013;2013:437613. doi: 10.1155/2013/437613. Epub 2013 Jul 14. Biomed Res Int. 2013. PMID: 23936799 Free PMC article. Review.
-
Idebenone improves motor dysfunction, learning and memory by regulating mitophagy in MPTP-treated mice.Cell Death Discov. 2022 Jan 17;8(1):28. doi: 10.1038/s41420-022-00826-8. Cell Death Discov. 2022. PMID: 35039479 Free PMC article.
-
Nutrient-sensing mTORC1: Integration of metabolic and autophagic signals.J Mol Cell Cardiol. 2016 Jun;95:31-41. doi: 10.1016/j.yjmcc.2016.01.005. Epub 2016 Jan 7. J Mol Cell Cardiol. 2016. PMID: 26773603 Free PMC article. Review.
References
-
- Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. - PubMed
-
- Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. - PubMed
-
- Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. - PubMed
-
- Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem. 2006;281:29776–29487. - PubMed
-
- Hamacher-Brady A, Brady NR, Logue SE, Sayen MR, Jinno M, Kirshenbaum LA, et al. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 2007;14:146–157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
