Neuroanatomical and neurochemical substrates of timing
- PMID: 20668434
- PMCID: PMC3055517
- DOI: 10.1038/npp.2010.113
Neuroanatomical and neurochemical substrates of timing
Abstract
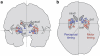
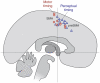
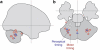
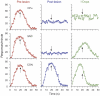
We all have a sense of time. Yet, there are no sensory receptors specifically dedicated for perceiving time. It is an almost uniquely intangible sensation: we cannot see time in the way that we see color, shape, or even location. So how is time represented in the brain? We explore the neural substrates of metrical representations of time such as duration estimation (explicit timing) or temporal expectation (implicit timing). Basal ganglia (BG), supplementary motor area, cerebellum, and prefrontal cortex have all been linked to the explicit estimation of duration. However, each region may have a functionally discrete role and will be differentially implicated depending upon task context. Among these, the dorsal striatum of the BG and, more specifically, its ascending nigrostriatal dopaminergic pathway seems to be the most crucial of these regions, as shown by converging functional neuroimaging, neuropsychological, and psychopharmacological investigations in humans, as well as lesion and pharmacological studies in animals. Moreover, neuronal firing rates in both striatal and interconnected frontal areas vary as a function of duration, suggesting a neurophysiological mechanism for the representation of time in the brain, with the excitatory-inhibitory balance of interactions among distinct subtypes of striatal neuron serving to fine-tune temporal accuracy and precision.
Figures

Similar articles
-
Dissociating explicit timing from temporal expectation with fMRI.Curr Opin Neurobiol. 2008 Apr;18(2):137-44. doi: 10.1016/j.conb.2008.07.011. Epub 2008 Aug 12. Curr Opin Neurobiol. 2008. PMID: 18692573 Review.
-
The neural representation of time.Curr Opin Neurobiol. 2004 Apr;14(2):225-32. doi: 10.1016/j.conb.2004.03.013. Curr Opin Neurobiol. 2004. PMID: 15082329 Review.
-
Dopaminergic Modulation of Synaptic Integration and Firing Patterns in the Rat Entopeduncular Nucleus.J Neurosci. 2017 Jul 26;37(30):7177-7187. doi: 10.1523/JNEUROSCI.0639-17.2017. Epub 2017 Jun 26. J Neurosci. 2017. PMID: 28652413 Free PMC article.
-
Dopamine precursor depletion impairs timing in healthy volunteers by attenuating activity in putamen and supplementary motor area.J Neurosci. 2012 Nov 21;32(47):16704-15. doi: 10.1523/JNEUROSCI.1258-12.2012. J Neurosci. 2012. PMID: 23175824 Free PMC article.
-
The roles of the cerebellum and basal ganglia in timing and error prediction.Eur J Neurosci. 2002 Oct;16(8):1609-19. doi: 10.1046/j.1460-9568.2002.02212.x. Eur J Neurosci. 2002. PMID: 12405975
Cited by
-
A neuropsychological approach to time estimation.Dialogues Clin Neurosci. 2012 Dec;14(4):425-32. doi: 10.31887/DCNS.2012.14.4/sphatif. Dialogues Clin Neurosci. 2012. PMID: 23393418 Free PMC article.
-
The neural bases for timing of durations.Nat Rev Neurosci. 2022 Nov;23(11):646-665. doi: 10.1038/s41583-022-00623-3. Epub 2022 Sep 12. Nat Rev Neurosci. 2022. PMID: 36097049 Review.
-
Statistical analysis of single-trial Granger causality spectra.Comput Math Methods Med. 2012;2012:697610. doi: 10.1155/2012/697610. Epub 2012 May 10. Comput Math Methods Med. 2012. PMID: 22649482 Free PMC article.
-
Rational inattention and tonic dopamine.PLoS Comput Biol. 2021 Mar 24;17(3):e1008659. doi: 10.1371/journal.pcbi.1008659. eCollection 2021 Mar. PLoS Comput Biol. 2021. PMID: 33760806 Free PMC article.
-
Neural population clocks: Encoding time in dynamic patterns of neural activity.Behav Neurosci. 2022 Oct;136(5):374-382. doi: 10.1037/bne0000515. Epub 2022 Apr 21. Behav Neurosci. 2022. PMID: 35446093 Free PMC article.
References
-
- Agid O, Mamo D, Ginovart N, Vitcu I, Wilson AA, Zipursky RB, et al. Striatal vs extrastriatal dopamine D2 receptors in antipsychotic response--a double-blind PET study in schizophrenia. Neuropsychopharmacology. 2007;32:1209–1215. - PubMed
-
- Akkal D, Escola L, Bioulac B, Burbaud P. Time predictability modulates pre-supplementary motor area neuronal activity. NeuroReport. 2004;15:1283–1286. - PubMed
-
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci. 1986;9:357–381. - PubMed
-
- Aosaki T, Kimura M, Graybiel AM. Temporal and spatial characteristics of tonically active neurons of the primate's striatum. J Neurophysiol. 1995;73:1234–1252. - PubMed
-
- Aparicio P, Diedrichsen J, Ivry RB. Effects of focal basal ganglia lesions on timing and force control. Brain Cogn. 2005;58:62–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

