Identification of roles for peptide: N-glycanase and endo-beta-N-acetylglucosaminidase (Engase1p) during protein N-glycosylation in human HepG2 cells
- PMID: 20668520
- PMCID: PMC2909182
- DOI: 10.1371/journal.pone.0011734
Identification of roles for peptide: N-glycanase and endo-beta-N-acetylglucosaminidase (Engase1p) during protein N-glycosylation in human HepG2 cells
Abstract
Background: During mammalian protein N-glycosylation, 20% of all dolichol-linked oligosaccharides (LLO) appear as free oligosaccharides (fOS) bearing the di-N-acetylchitobiose (fOSGN2), or a single N-acetylglucosamine (fOSGN), moiety at their reducing termini. After sequential trimming by cytosolic endo beta-N-acetylglucosaminidase (ENGase) and Man2c1 mannosidase, cytosolic fOS are transported into lysosomes. Why mammalian cells generate such large quantities of fOS remains unexplored, but fOSGN2 could be liberated from LLO by oligosaccharyltransferase, or from glycoproteins by NGLY1-encoded Peptide-N-Glycanase (PNGase). Also, in addition to converting fOSGN2 to fOSGN, the ENGASE-encoded cytosolic ENGase of poorly defined function could potentially deglycosylate glycoproteins. Here, the roles of Ngly1p and Engase1p during fOS metabolism were investigated in HepG2 cells.
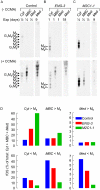
Methods/principal findings: During metabolic radiolabeling and chase incubations, RNAi-mediated Engase1p down regulation delays fOSGN2-to-fOSGN conversion, and it is shown that Engase1p and Man2c1p are necessary for efficient clearance of cytosolic fOS into lysosomes. Saccharomyces cerevisiae does not possess ENGase activity and expression of human Engase1p in the png1Delta deletion mutant, in which fOS are reduced by over 98%, partially restored fOS generation. In metabolically radiolabeled HepG2 cells evidence was obtained for a small but significant Engase1p-mediated generation of fOS in 1 h chase but not 30 min pulse incubations. Ngly1p down regulation revealed an Ngly1p-independent fOSGN2 pool comprising mainly Man(8)GlcNAc(2), corresponding to approximately 70% of total fOS, and an Ngly1p-dependent fOSGN2 pool enriched in Glc(1)Man(9)GlcNAc(2) and Man(9)GlcNAc(2) that corresponds to approximately 30% of total fOS.
Conclusions/significance: As the generation of the bulk of fOS is unaffected by co-down regulation of Ngly1p and Engase1p, alternative quantitatively important mechanisms must underlie the liberation of these fOS from either LLO or glycoproteins during protein N-glycosylation. The fully mannosylated structures that occur in the Ngly1p-dependent fOSGN2 pool indicate an ERAD process that does not require N-glycan trimming.
Conflict of interest statement
Figures


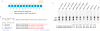

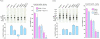

References
-
- Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. - PubMed
-
- Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science. 2001;291:2364–2369. - PubMed
-
- Chantret I, Moore SE. Free oligosaccharide regulation during mammalian protein N-glycosylation. Glycobiology. 2008;18:210–224. - PubMed
-
- Anumula KR, Spiro RG. Release of glucose-containing polymannose oligosaccharides during glycoprotein biosynthesis. Studies with thyroid microsomal enzymes and slices. J Biol Chem. 1983;258:15274–15282. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

