Persistence of pathogens with short infectious periods in seasonal tick populations: the relative importance of three transmission routes
- PMID: 20668521
- PMCID: PMC2909195
- DOI: 10.1371/journal.pone.0011745
Persistence of pathogens with short infectious periods in seasonal tick populations: the relative importance of three transmission routes
Abstract
Background: The flaviviruses causing tick-borne encephalitis (TBE) persist at low but consistent levels in tick populations, despite short infectious periods in their mammalian hosts and transmission periods constrained by distinctly seasonal tick life cycles. In addition to systemic and vertical transmission, cofeeding transmission has been proposed as an important route for the persistence of TBE-causing viruses. Because cofeeding transmission requires ticks to feed simultaneously, the timing of tick activity may be critical to pathogen persistence. Existing models of tick-borne diseases do not incorporate all transmission routes and tick seasonality. Our aim is to evaluate the influence of seasonality on the relative importance of different transmission routes by using a comprehensive mathematical model.
Methodology/principal findings: We developed a stage-structured population model that includes tick seasonality and evaluated the relative importance of the transmission routes for pathogens with short infectious periods, in particular Powassan virus (POWV) and the related "deer tick virus," emergent encephalitis-causing flaviviruses in North America. We used the next generation matrix method to calculate the basic reproductive ratio and performed elasticity analyses. We confirmed that cofeeding transmission is critically important for such pathogens to persist in seasonal tick populations over the reasonable range of parameter values. At higher but still plausible rates of vertical transmission, our model suggests that vertical transmission can strongly enhance pathogen prevalence when it operates in combination with cofeeding transmission.
Conclusions/significance: Our results demonstrate that the consistent prevalence of POWV observed in tick populations could be maintained by a combination of low vertical, intermediate cofeeding and high systemic transmission rates. When vertical transmission is weak, nymphal ticks support integral parts of the transmission cycle that are critical for maintaining the pathogen. We also extended the model to pathogens that cause chronic infections in hosts and found that cofeeding transmission could contribute to elevating prevalence even in these systems. Therefore, the common assumption that cofeeding transmission is not relevant in models of chronic host infection, such as Lyme disease, could lead to underestimating pathogen prevalence.
Conflict of interest statement
Figures
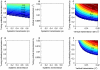
 = 0; A, D), without cofeeding transmission (all
= 0; A, D), without cofeeding transmission (all  's = 0; B, E), and without systemic transmission (
's = 0; B, E), and without systemic transmission ( and
and  = 0; C, F). The blank colour means prevalence = 0. Cofeeding transmission is expressed as the multiples of the baseline rates (0.24 and 0.72). Neither vertical nor systemic transmission along can maintain the pathogen, while cofeeding transmission is necessary and can be sufficient by itself to sustain the pathogen in the tick population. The corresponding figures for R0 values are presented in Fig. S2.
= 0; C, F). The blank colour means prevalence = 0. Cofeeding transmission is expressed as the multiples of the baseline rates (0.24 and 0.72). Neither vertical nor systemic transmission along can maintain the pathogen, while cofeeding transmission is necessary and can be sufficient by itself to sustain the pathogen in the tick population. The corresponding figures for R0 values are presented in Fig. S2.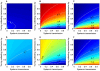
 ) = 0.001, with inter-cohort overlap (A, B, C) and without (D, E, F). In both cases, relative importance of the three routes varies over the parameter space, notably cofeeding and systemic transmission exchanging relative importance as the baseline cofeeding transmission rate increases (Fig. 3B, C, E, F). Cohort overlap did not qualitatively change the results, but vertical transmission increased its relative importance by 10–15% (Fig. 3A,D).
) = 0.001, with inter-cohort overlap (A, B, C) and without (D, E, F). In both cases, relative importance of the three routes varies over the parameter space, notably cofeeding and systemic transmission exchanging relative importance as the baseline cofeeding transmission rate increases (Fig. 3B, C, E, F). Cohort overlap did not qualitatively change the results, but vertical transmission increased its relative importance by 10–15% (Fig. 3A,D).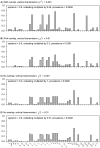
 ) has high elasticity, and it is dominated by the pathway from nymphs to larvae (
) has high elasticity, and it is dominated by the pathway from nymphs to larvae ( ). Case 2 – higher vertical and lower cofeeding transmission – the parameters for larvae increase elasticities.
). Case 2 – higher vertical and lower cofeeding transmission – the parameters for larvae increase elasticities.


Similar articles
-
Tick-borne encephalitis virus transmission between ticks cofeeding on specific immune natural rodent hosts.Virology. 1997 Aug 18;235(1):138-43. doi: 10.1006/viro.1997.8622. Virology. 1997. PMID: 9300045
-
Incidence from coincidence: patterns of tick infestations on rodents facilitate transmission of tick-borne encephalitis virus.Parasitology. 1999 Feb;118 ( Pt 2):177-86. doi: 10.1017/s0031182098003643. Parasitology. 1999. PMID: 10028532
-
Incorporating tick feeding behaviour into R0 for tick-borne pathogens.Theor Popul Biol. 2020 Feb;131:25-37. doi: 10.1016/j.tpb.2019.10.004. Epub 2019 Nov 12. Theor Popul Biol. 2020. PMID: 31730874 Free PMC article.
-
Transmission of tick-borne pathogens between co-feeding ticks: Milan Labuda's enduring paradigm.Ticks Tick Borne Dis. 2011 Dec;2(4):179-82. doi: 10.1016/j.ttbdis.2011.07.004. Epub 2011 Sep 16. Ticks Tick Borne Dis. 2011. PMID: 22108009 Review.
-
Seasonal population dynamics of ixodes ticks and tick-borne encephalitis virus.Exp Appl Acarol. 2000;24(9):665-81. doi: 10.1023/a:1010798518261. Exp Appl Acarol. 2000. PMID: 11227825 Review.
Cited by
-
Prevalence of tick borne encephalitis virus in tick nymphs in relation to climatic factors on the southern coast of Norway.Parasit Vectors. 2012 Aug 22;5:177. doi: 10.1186/1756-3305-5-177. Parasit Vectors. 2012. PMID: 22913287 Free PMC article.
-
The known unknowns of Powassan virus ecology.J Med Entomol. 2023 Nov 14;60(6):1142-1148. doi: 10.1093/jme/tjad095. J Med Entomol. 2023. PMID: 37862099 Free PMC article. Review.
-
First evidence of transovarial transmission of Kyasanur Forest disease virus in Haemaphysalis and Rhipicephalus ticks in the wild.Parasit Vectors. 2025 Jan 17;18(1):14. doi: 10.1186/s13071-024-06643-5. Parasit Vectors. 2025. PMID: 39825388 Free PMC article.
-
The tortoise or the hare? Impacts of within-host dynamics on transmission success of arthropod-borne viruses.Philos Trans R Soc Lond B Biol Sci. 2015 Aug 19;370(1675):20140299. doi: 10.1098/rstb.2014.0299. Philos Trans R Soc Lond B Biol Sci. 2015. PMID: 26150665 Free PMC article. Review.
-
Impact of life stage-dependent dispersal on the colonization dynamics of host patches by ticks and tick-borne infectious agents.Parasit Vectors. 2017 Aug 4;10(1):375. doi: 10.1186/s13071-017-2261-y. Parasit Vectors. 2017. PMID: 28778181 Free PMC article.
References
-
- Sumilo D, Bormane A, Asokliene L, Vasilenko V, Golovjova I, et al. Socio-economic factors in the differential upsurge of tick-borne encephalitis in central and eastern Europe. Reviews in Medical Virology. 2008;18:81–95. - PubMed
-
- Süss J. Tick-borne encephalitis in Europe and beyond – the epidemiological situation as of 2007. Eurosurveillance. 2008;13:1–8. - PubMed
-
- Mansfield KL, Johnson N, Phipps LP, Stephenson JR, Fooks AR, et al. Tick-borne encephalitis virus - a review of an emerging zoonosis. Journal of General Virology. 2009;90:1781–1794. - PubMed
-
- Hinten SR, Beckett GA, Gensheimer KF, Pritchard E, Courtney TM, et al. Increased recognition of Powassan encephalitis in the United States, 1999–2005. Vector-borne and Zoonotic Diseases. 2008;8:733–740. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

