Human microglia transplanted in rat focal ischemia brain induce neuroprotection and behavioral improvement
- PMID: 20668522
- PMCID: PMC2909196
- DOI: 10.1371/journal.pone.0011746
Human microglia transplanted in rat focal ischemia brain induce neuroprotection and behavioral improvement
Abstract
Background and purpose: Microglia are resident immunocompetent and phagocytic cells of central nervous system (CNS), which produce various cytokines and growth factors in response to injury and thereby regulate disease pathology. The purpose of this study is to investigate the effects of microglial transplantation on focal cerebral ischemia model in rat.
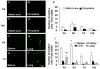
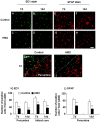
Methods: Transient middle cerebral artery occlusion (MCAO) in rats was induced by the intraluminal filament technique. HMO6 cells, human microglial cell line, were transplanted intravenously at 48 hours after MCAO. Functional tests were performed and the infarct volume was measured at 7 and 14 days after MCAO. Migration and cell survival of transplanted microglial cells and host glial reaction in the brain were studied by immunohistochemistry. Gene expression of neurotrophic factors, cytokines and chemokines in transplanted cells and host rat glial cells was determined by laser capture microdissection (LCM) and quantitative real time-PCR.
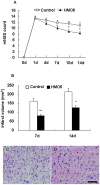
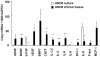
Results: HMO6 human microglial cells transplantation group demonstrated significant functional recovery compared with control group. At 7 and 14 days after MCAO, infarct volume was significantly reduced in the HMO group. In the HMO6 group, number of apoptotic cells was time-dependently reduced in the infarct core and penumbra. In addition, number of host rat microglia/macrophages and reactive astrocytes was significantly decreased at 7 and 14 days after MCAO in the penumbra. Gene expression of various neurotrophic factors (GDNF, BDNF, VEGF and BMP7) and anti-inflammatory cytokines (IL4 and IL5) was up-regulated in transplanted HMO6 cells of brain tissue compared with those in culture. The expression of GDNF and VEGF in astrocytes in penumbra was significantly up-regulated in the HMO6 group.
Conclusions: Our results indicate that transplantation of HMO6 human microglial cells reduces ischemic deficits and apoptotic events in stroke animals. The results were mediated by modulation of gliosis and neuroinflammation, and neuroprotection provided by neurotrophic factors of endogenous and transplanted cells-origin.
Conflict of interest statement
Figures

Similar articles
-
Transplantation of human mesenchymal stem cells promotes functional improvement and increased expression of neurotrophic factors in a rat focal cerebral ischemia model.J Neurosci Res. 2010 Apr;88(5):1017-25. doi: 10.1002/jnr.22279. J Neurosci Res. 2010. PMID: 19885863
-
A Mesenchymal stem cell line (B10) increases angiogenesis in a rat MCAO model.Exp Neurol. 2019 Jan;311:182-193. doi: 10.1016/j.expneurol.2018.10.001. Epub 2018 Oct 3. Exp Neurol. 2019. PMID: 30291853
-
Intravenously Delivered Allogeneic Mesenchymal Stem Cells Bidirectionally Regulate Inflammation and Induce Neurotrophic Effects in Distal Middle Cerebral Artery Occlusion Rats Within the First 7 Days After Stroke.Cell Physiol Biochem. 2018;46(5):1951-1970. doi: 10.1159/000489384. Epub 2018 Apr 26. Cell Physiol Biochem. 2018. PMID: 29719282
-
Recent Progress in Therapeutic Strategies for Ischemic Stroke.Cell Transplant. 2016;25(5):893-8. doi: 10.3727/096368916X690548. Epub 2016 Jan 18. Cell Transplant. 2016. PMID: 26786838 Review.
-
Microglia as the Critical Regulators of Neuroprotection and Functional Recovery in Cerebral Ischemia.Cell Mol Neurobiol. 2022 Nov;42(8):2505-2525. doi: 10.1007/s10571-021-01145-9. Epub 2021 Aug 30. Cell Mol Neurobiol. 2022. PMID: 34460037 Free PMC article. Review.
Cited by
-
Acupuncture Alleviates Chronic Ischemic White Matter Injury in SHR Rats via JNK-NMDAR Circuit.Mol Neurobiol. 2024 Jun;61(6):3144-3160. doi: 10.1007/s12035-023-03759-0. Epub 2023 Nov 17. Mol Neurobiol. 2024. PMID: 37976026
-
Effect of δ-opioid receptor activation on BDNF-TrkB vs. TNF-α in the mouse cortex exposed to prolonged hypoxia.Int J Mol Sci. 2013 Jul 31;14(8):15959-76. doi: 10.3390/ijms140815959. Int J Mol Sci. 2013. PMID: 23912236 Free PMC article.
-
Neurovascular Unit as a Source of Ischemic Stroke Biomarkers-Limitations of Experimental Studies and Perspectives for Clinical Application.Transl Stroke Res. 2020 Aug;11(4):553-579. doi: 10.1007/s12975-019-00744-5. Epub 2019 Nov 7. Transl Stroke Res. 2020. PMID: 31701356 Free PMC article. Review.
-
Neuroinflammation as a Key Driver of Secondary Neurodegeneration Following Stroke?Int J Mol Sci. 2021 Dec 3;22(23):13101. doi: 10.3390/ijms222313101. Int J Mol Sci. 2021. PMID: 34884906 Free PMC article. Review.
-
Potential therapeutic effects of neurotrophins for acute and chronic neurological diseases.Biomed Res Int. 2014;2014:601084. doi: 10.1155/2014/601084. Epub 2014 Apr 9. Biomed Res Int. 2014. PMID: 24818146 Free PMC article. Review.
References
-
- Gonzalez-Scarano F, Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Annu Rev Neurosci. 1999;22:219–240. - PubMed
-
- Kim SU, de Vellis J. Microglia in health and disease. J Neurosci Res. 2005;81:302–313. - PubMed
-
- Lee YB, Nagai A, Kim SU. Cytokines, chemokines, and cytokine receptors in human microglia. J Neurosci Res. 2002;69:94–103. - PubMed
-
- McGeer PL, McGeer EG. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Rev. 1995;21:195–218. - PubMed
-
- Block F, Peters M, Nolden-Koch M. Expression of IL-6 in the ischemic penumbra. Neuroreport. 2000;11:963–967. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

