Role of the ATM-checkpoint kinase 2 pathway in CDT-mediated apoptosis of gingival epithelial cells
- PMID: 20668524
- PMCID: PMC2909199
- DOI: 10.1371/journal.pone.0011714
Role of the ATM-checkpoint kinase 2 pathway in CDT-mediated apoptosis of gingival epithelial cells
Abstract
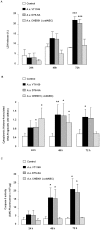
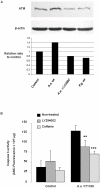
The cytolethal distending toxin (CDT) of the oral pathogen Aggregatibacter actinomycetemcomitans induces cell cycle arrest and apoptosis in various cell types. Western analysis, pharmacological inhibition and siRNA silencing were performed in human immortalized gingival keratinocytes (HIGK) to dissect the functional role of the ataxia telangiectasia mutated (ATM) pathway in the signal transduction steps triggered by the CDT. Infection of HIGK was associated with a time-dependent induction of cytoplasmic histone-associated DNA fragmentation. However, in the absence of CDT, infected HIGK underwent reversible DNA strand breaks but not apoptosis, while caspase 3 activity, p21 levels, and HIGK viability were unaffected. Caspase 9 activity was attenuated in the CDT mutant-infected HIGK compared to wild-type infected cells. Pharmacological inhibition and siRNA-silencing of the ATM downstream effector, the protein kinase checkpoint kinase 2 (Chk2), significantly impacted CDT-mediated apoptosis. Together, these findings provide insight on the specificity of the ATM-Chk2 pathway in response to the CDT of A. actinomycetemcomitans in oral epithelial cells, which ultimately leads to apoptosis. We further propose the existence of an unidentified factor that is distinct from the CDT, and involved with a reversible DNA fragmentation that does not trigger terminal apoptosis in oral epithelial cells. This model potentially explains conflicting reports on the biological activity of the A. actinomycetemcomitans CDT.
Conflict of interest statement
Figures



Similar articles
-
The Haemophilus ducreyi cytolethal distending toxin induces cell cycle arrest and apoptosis via the DNA damage checkpoint pathways.J Biol Chem. 2001 Feb 16;276(7):5296-302. doi: 10.1074/jbc.M008527200. Epub 2000 Nov 13. J Biol Chem. 2001. PMID: 11076947
-
Chk1 is dispensable for G2 arrest in response to sustained DNA damage when the ATM/p53/p21 pathway is functional.Oncogene. 2011 Oct 13;30(41):4261-74. doi: 10.1038/onc.2011.135. Epub 2011 May 2. Oncogene. 2011. PMID: 21532626
-
Gallic acid causes inactivating phosphorylation of cdc25A/cdc25C-cdc2 via ATM-Chk2 activation, leading to cell cycle arrest, and induces apoptosis in human prostate carcinoma DU145 cells.Mol Cancer Ther. 2006 Dec;5(12):3294-302. doi: 10.1158/1535-7163.MCT-06-0483. Mol Cancer Ther. 2006. PMID: 17172433
-
Activation and regulation of ATM kinase activity in response to DNA double-strand breaks.Oncogene. 2007 Dec 10;26(56):7741-8. doi: 10.1038/sj.onc.1210872. Oncogene. 2007. PMID: 18066086 Review.
-
Cytolethal distending toxin: a conserved bacterial genotoxin that blocks cell cycle progression, leading to apoptosis of a broad range of mammalian cell lineages.Microbiology (Reading). 2011 Jul;157(Pt 7):1851-1875. doi: 10.1099/mic.0.049536-0. Epub 2011 May 12. Microbiology (Reading). 2011. PMID: 21565933 Free PMC article. Review.
Cited by
-
A new functional site W115 in CdtA is critical for Aggregatibacter actinomycetemcomitans cytolethal distending toxin.PLoS One. 2013 Jun 3;8(6):e65729. doi: 10.1371/journal.pone.0065729. Print 2013. PLoS One. 2013. PMID: 23755273 Free PMC article.
-
Cell resistance to the Cytolethal Distending Toxin involves an association of DNA repair mechanisms.Sci Rep. 2016 Oct 24;6:36022. doi: 10.1038/srep36022. Sci Rep. 2016. PMID: 27775089 Free PMC article.
-
Mini but mighty: microRNAs in the pathobiology of periodontal disease.Periodontol 2000. 2015 Oct;69(1):201-20. doi: 10.1111/prd.12095. Periodontol 2000. 2015. PMID: 26252410 Free PMC article. Review.
-
Sensitization of radio-resistant prostate cancer cells with a unique cytolethal distending toxin.Oncotarget. 2014 Jul 30;5(14):5523-34. doi: 10.18632/oncotarget.2133. Oncotarget. 2014. PMID: 25015118 Free PMC article.
-
A type I IFN-dependent DNA damage response regulates the genetic program and inflammasome activation in macrophages.Elife. 2017 Mar 31;6:e24655. doi: 10.7554/eLife.24655. Elife. 2017. PMID: 28362262 Free PMC article.
References
-
- Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78–111. - PubMed
-
- van Winkelhoff AJ, Slots J. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in nonoral infections. Periodontol 2000. 1999;20:122–135. - PubMed
-
- Offenbacher S, Jared HL, O'Reilly PG, Wells SR, Salvi GE, et al. Potential pathogenic mechanisms of periodontitis associated pregnancy complications. Ann Periodontol. 1998;3:233–250. - PubMed
-
- Marques da Silva R, Caugant DA, Lingaas PS, Geiran O, Tronstad L, et al. Detection of Actinobacillus actinomycetemcomitans but not bacteria of the red complex in aortic aneurysms by multiplex polymerase chain reaction. J Periodontol. 2005;76:590–594. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

