Development and validation of a quantitative, high-throughput, fluorescent-based bioassay to detect schistosoma viability
- PMID: 20668553
- PMCID: PMC2910722
- DOI: 10.1371/journal.pntd.0000759
Development and validation of a quantitative, high-throughput, fluorescent-based bioassay to detect schistosoma viability
Abstract
Background: Schistosomiasis, caused by infection with the blood fluke Schistosoma, is responsible for greater than 200,000 human deaths per annum. Objective high-throughput screens for detecting novel anti-schistosomal targets will drive 'genome to drug' lead translational science at an unprecedented rate. Current methods for detecting schistosome viability rely on qualitative microscopic criteria, which require an understanding of parasite morphology, and most importantly, must be subjectively interpreted. These limitations, in the current state of the art, have significantly impeded progress into whole schistosome screening for next generation chemotherapies.
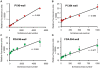
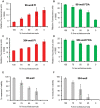
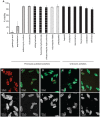
Methodology/principal findings: We present here a microtiter plate-based method for reproducibly detecting schistosomula viability that takes advantage of the differential uptake of fluorophores (propidium iodide and fluorescein diacetate) by living organisms. We validate this high-throughput system in detecting schistosomula viability using auranofin (a known inhibitor of thioredoxin glutathione reductase), praziquantel and a range of small compounds with previously-described (gambogic acid, sodium salinomycin, ethinyl estradiol, fluoxetidine hydrochloride, miconazole nitrate, chlorpromazine hydrochloride, amphotericin b, niclosamide) or suggested (bepridil, ciclopirox, rescinnamine, flucytosine, vinblastine and carbidopa) anti-schistosomal activities. This developed method is sensitive (200 schistosomula/well can be assayed), relevant to industrial (384-well microtiter plate compatibility) and academic (96-well microtiter plate compatibility) settings, translatable to functional genomics screens and drug assays, does not require a priori knowledge of schistosome biology and is quantitative.
Conclusions/significance: The wide-scale application of this fluorescence-based bioassay will greatly accelerate the objective identification of novel therapeutic lead targets/compounds to combat schistosomiasis. Adapting this bioassay for use with other parasitic worm species further offers an opportunity for great strides to be made against additional neglected tropical diseases of biomedical and veterinary importance.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. J Lancet. 2005;365:1561–1569. - PubMed
-
- Fenwick A, Savioli L, Engels D, Robert Bergquist N, Todd MH. Drugs for the control of parasitic diseases: current status and development in schistosomiasis. Trends Parasitol. 2003;19:509–515. - PubMed
-
- Brindley PJ, Pearce EJ. Genetic manipulation of schistosomes. Int J for Parasitol. 2007;37:465–473. - PubMed
-
- Hokke CH, Fitzpatrick JM, Hoffmann KF. Integrating transcriptome, proteome and glycome analyses of Schistosoma biology. Trends Parasitol. 2007;23:165–174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

