Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation
- PMID: 20668705
- PMCID: PMC2909263
- DOI: 10.1371/journal.pone.0011765
Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation
Abstract
Background: Chronic inflammation of the arterial wall is a key element in the pathogenesis of atherosclerosis, yet the factors that trigger and sustain the inflammation remain elusive. Inflammasomes are cytoplasmic caspase-1-activating protein complexes that promote maturation and secretion of the proinflammatory cytokines interleukin(IL)-1beta and IL-18. The most intensively studied inflammasome, NLRP3 inflammasome, is activated by diverse substances, including crystalline and particulate materials. As cholesterol crystals are abundant in atherosclerotic lesions, and IL-1beta has been linked to atherogenesis, we explored the possibility that cholesterol crystals promote inflammation by activating the inflammasome pathway.
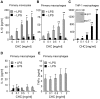
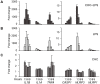
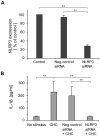
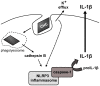
Principal findings: Here we show that human macrophages avidly phagocytose cholesterol crystals and store the ingested cholesterol as cholesteryl esters. Importantly, cholesterol crystals induced dose-dependent secretion of mature IL-1beta from human monocytes and macrophages. The cholesterol crystal-induced secretion of IL-1beta was caspase-1-dependent, suggesting the involvement of an inflammasome-mediated pathway. Silencing of the NLRP3 receptor, the crucial component in NLRP3 inflammasome, completely abolished crystal-induced IL-1beta secretion, thus identifying NLRP3 inflammasome as the cholesterol crystal-responsive element in macrophages. The crystals were shown to induce leakage of the lysosomal protease cathepsin B into the cytoplasm and inhibition of this enzyme reduced cholesterol crystal-induced IL-1beta secretion, suggesting that NLRP3 inflammasome activation occurred via lysosomal destabilization.
Conclusions: The cholesterol crystal-induced inflammasome activation in macrophages may represent an important link between cholesterol metabolism and inflammation in atherosclerotic lesions.
Conflict of interest statement
Figures



References
-
- Tedgui A, Mallat Z. Cytokines in atherosclerosis: Pathogenic and regulatory pathways. Physiol Rev. 2006;86(2):515–581. - PubMed
-
- Galea J, Armstrong J, Gadsdon P, Holden H, Francis SE, et al. Interleukin-1 beta in coronary arteries of patients with ischemic heart disease. Arterioscler Thromb Vasc Biol. 1996;16(8):1000–1006. - PubMed
-
- Kirii H, Niwa T, Yamada Y, Wada H, Saito K, et al. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23(4):656–660. - PubMed
-
- Wang X, Feuerstein GZ, Gu JL, Lysko PG, Yue TL. Interleukin-1 beta induces expression of adhesion molecules in human vascular smooth muscle cells and enhances adhesion of leukocytes to smooth muscle cells. Atherosclerosis. 1995;115(1):89–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

