Structure-activity relationships of a small-molecule inhibitor of the PDZ domain of PICK1
- PMID: 20668766
- PMCID: PMC3614091
- DOI: 10.1039/c0ob00025f
Structure-activity relationships of a small-molecule inhibitor of the PDZ domain of PICK1
Abstract
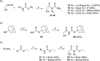
Recently, we described the first small-molecule inhibitor, (E)-ethyl 2-cyano-3-(3,4-dichlorophenyl)acryloylcarbamate (1), of the PDZ domain of protein interacting with Calpha-kinase 1 (PICK1), a potential drug target against brain ischemia, pain and cocaine addiction. Herein, we explore structure-activity relationships of 1 by introducing subtle modifications of the acryloylcarbamate scaffold and variations of the substituents on this scaffold. The configuration around the double bond of 1 and analogues was settled by a combination of X-ray crystallography, NMR and density functional theory calculations. Thereby, docking studies were used to correlate biological affinities with structural considerations for ligand-protein interactions. The most potent analogue obtained in this study showed an improvement in affinity compared to 1 and is currently a lead in further studies of PICK1 inhibition.
Figures
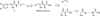


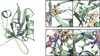


Similar articles
-
Lock and chop: A novel method for the generation of a PICK1 PDZ domain and piperidine-based inhibitor co-crystal structure.Protein Sci. 2018 Mar;27(3):672-680. doi: 10.1002/pro.3361. Epub 2018 Jan 30. Protein Sci. 2018. PMID: 29280296 Free PMC article.
-
Identification of a small-molecule inhibitor of the PICK1 PDZ domain that inhibits hippocampal LTP and LTD.Proc Natl Acad Sci U S A. 2010 Jan 5;107(1):413-8. doi: 10.1073/pnas.0902225107. Epub 2009 Dec 14. Proc Natl Acad Sci U S A. 2010. PMID: 20018661 Free PMC article.
-
The binding affinities of proteins interacting with the PDZ domain of PICK1.Proteins. 2012 May;80(5):1393-408. doi: 10.1002/prot.24034. Epub 2012 Feb 24. Proteins. 2012. PMID: 22275068
-
The schizophrenic faces of PICK1.Trends Pharmacol Sci. 2006 Nov;27(11):574-9. doi: 10.1016/j.tips.2006.09.007. Epub 2006 Sep 29. Trends Pharmacol Sci. 2006. PMID: 17011050 Free PMC article. Review.
-
Protein interacting with C kinase and neurological disorders.Synapse. 2013 Aug;67(8):532-40. doi: 10.1002/syn.21657. Epub 2013 Mar 20. Synapse. 2013. PMID: 23436724 Review.
Cited by
-
A high-affinity, bivalent PDZ domain inhibitor complexes PICK1 to alleviate neuropathic pain.EMBO Mol Med. 2020 Jun 8;12(6):e11248. doi: 10.15252/emmm.201911248. Epub 2020 Apr 30. EMBO Mol Med. 2020. PMID: 32352640 Free PMC article.
-
Liposomal Encapsulated FSC231, a PICK1 Inhibitor, Prevents the Ischemia/Reperfusion-Induced Degradation of GluA2-Containing AMPA Receptors.Pharmaceutics. 2021 Apr 30;13(5):636. doi: 10.3390/pharmaceutics13050636. Pharmaceutics. 2021. PMID: 33946313 Free PMC article.
-
Structure function relations in PDZ-domain-containing proteins: Implications for protein networks in cellular signalling.J Biosci. 2018 Mar;43(1):155-171. J Biosci. 2018. PMID: 29485124 Review.
-
PDZ Domains as Drug Targets.Adv Ther (Weinh). 2019 Jul;2(7):1800143. doi: 10.1002/adtp.201800143. Epub 2019 Apr 24. Adv Ther (Weinh). 2019. PMID: 32313833 Free PMC article. Review.
-
Targeting receptor complexes: a new dimension in drug discovery.Nat Rev Drug Discov. 2020 Dec;19(12):884-901. doi: 10.1038/s41573-020-0086-4. Epub 2020 Nov 11. Nat Rev Drug Discov. 2020. PMID: 33177699 Review.
References
-
- Arkin MR, Wells JA. Nat. Rev. Drug Discovery. 2004;3:301–317. - PubMed
-
- Wells JA, McClendon CL. Nature. 2007;450:1001–1009. - PubMed
-
- Berg T. Curr. Opin. Drug Discovery Dev. 2008;11:666–674. - PubMed
-
- Dev KK. Nat. Rev. Drug Discovery. 2004;3:1047–1056. - PubMed
-
- Blazer LL, Neubig RR. Neuropsychopharmacology. 2009;34:126–141. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

