ATP and its N⁶-substituted analogues: parameterization, molecular dynamics simulation and conformational analysis
- PMID: 20668896
- PMCID: PMC3096017
- DOI: 10.1007/s00894-010-0808-3
ATP and its N⁶-substituted analogues: parameterization, molecular dynamics simulation and conformational analysis
Abstract
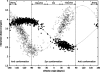
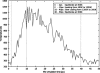
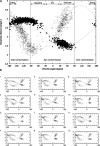
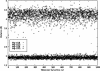
In this work we used a combination of classical molecular dynamics and simulated annealing techniques to shed more light on the conformational flexibility of 12 adenosine triphosphate (ATP) analogues in a water environment. We present simulations in AMBER force field for ATP and 12 published analogues [Shah et al. (1997) Proc Natl Acad Sci USA 94: 3565-3570]. The calculations were carried out using the generalized Born (GB) solvation model in the presence of the cation Mg(2+). The ion was placed at a close distance (2 Å) from the charged oxygen atoms of the beta and gamma phosphate groups of the -3 negatively charged ATP analogue molecules. Analysis of the results revealed the distribution of inter-proton distances H8-H1' and H8-H2' versus the torsion angle ψ (C4-N9-C1'-O4') for all conformations of ATP analogues. There are two gaps in the distribution of torsion angle ψ values: the first is between -30 and 30 degrees and is described by cis-conformation; and the second is between 90 and 175 degrees, which mostly covers a region of anti conformation. Our results compare favorably with results obtained in experimental assays [Jiang and Mao (2002) Polyhedron 21:435-438].
Figures

References
-
- Burnett G, Kennedy EP. The enzymatic phosphorylation of proteins. J Biol Chem. 1954;211:969–980. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

