Kinetic isotope effects in asymmetric reactions
- PMID: 20669194
- PMCID: PMC2996239
- DOI: 10.1002/chem.201001018
Kinetic isotope effects in asymmetric reactions
Abstract
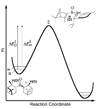
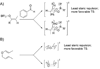
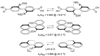
Kinetic isotope effects are exquisitely sensitive probes of transition structure. As such, kinetic isotope effects offer a uniquely useful probe for the symmetry-breaking process that is inherent to stereoselective reactions. In this Concept article, we explore the role of steric and electronic effects in stereocontrol, and we relate these concepts to recent studies carried out in our laboratory. We also explore the way in which kinetic isotope effects serve as useful points of contact with computational models of transition structures. Finally, we discuss future opportunities for kinetic isotope effects to play a role in asymmetric catalyst development.
Figures


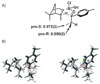












Similar articles
-
Computer-aided design of chiral ligands. Part 2. Functionality mapping as a method to identify stereocontrol elements for asymmetric reactions.J Org Chem. 2003 Mar 21;68(6):2061-76. doi: 10.1021/jo020401s. J Org Chem. 2003. PMID: 12636364
-
Stereocontrol in N-directed hydroboration: synthesis of amino alcohols related to the piperidine alkaloids.Org Lett. 2009 Mar 5;11(5):1059-61. doi: 10.1021/ol802781c. Org Lett. 2009. PMID: 19182927 Free PMC article.
-
Catalytic enantioselective diboration, disilation and silaboration: new opportunities for asymmetric synthesis.Chem Commun (Camb). 2007 Dec 7;(45):4717-25. doi: 10.1039/b707779c. Epub 2007 Aug 22. Chem Commun (Camb). 2007. PMID: 18004420 Review.
-
Isotope effects and the nature of enantioselectivity in the shi epoxidation. The importance of asynchronicity.J Am Chem Soc. 2005 May 11;127(18):6679-85. doi: 10.1021/ja0435788. J Am Chem Soc. 2005. PMID: 15869289
-
Homologation and alkylation of boronic esters and boranes by 1,2-metallate rearrangement of boronate complexes.Chem Rec. 2009;9(1):24-39. doi: 10.1002/tcr.20168. Chem Rec. 2009. PMID: 19243084 Review.
Cited by
-
Preparation of Deuterium-Labeled Armodafinil by Hydrogen-Deuterium Exchange and Its Application in Quantitative Analysis by LC-MS.Metabolites. 2022 Jun 22;12(7):578. doi: 10.3390/metabo12070578. Metabolites. 2022. PMID: 35888702 Free PMC article.
-
London Dispersion as a Design Element in Molecular Catalysis.J Am Chem Soc. 2025 Aug 20;147(33):29611-29623. doi: 10.1021/jacs.5c09212. Epub 2025 Aug 11. J Am Chem Soc. 2025. PMID: 40789051 Free PMC article. Review.
-
Assignment of the absolute configuration of molecules that are chiral by virtue of deuterium substitution using chiral tag molecular rotational resonance spectroscopy.Chirality. 2023 Nov;35(11):856-883. doi: 10.1002/chir.23596. Epub 2023 Jun 5. Chirality. 2023. PMID: 37277968 Free PMC article.
-
From bifunctional to trifunctional (tricomponent nucleophile-transition metal-lewis acid) catalysis: the catalytic, enantioselective α-fluorination of acid chlorides.J Am Chem Soc. 2011 May 18;133(19):7536-46. doi: 10.1021/ja2014345. Epub 2011 Apr 22. J Am Chem Soc. 2011. PMID: 21513338 Free PMC article.
-
Control of chemoselectivity in asymmetric tandem reactions: Direct synthesis of chiral amines bearing nonadjacent stereocenters.Proc Natl Acad Sci U S A. 2018 Feb 20;115(8):1730-1735. doi: 10.1073/pnas.1718474115. Epub 2018 Feb 5. Proc Natl Acad Sci U S A. 2018. PMID: 29432161 Free PMC article.
References
-
-
Recent examples: McCusker KP, Klinman JP. J. Am. Chem. Soc. 2010;132:5114–5120. Findlater M, Bernskoetter WH, Brookhart M. J. Am. Chem. Soc. 2010;132:4534–4535. Yasutomi Y, Suematsu H, Katsuki T. J. Am. Chem. Soc. 2010;132:4510–4511. Yang X, Zhao L, Fox T, Wang Z-X, Berke H. Angew. Chem. Int. Ed. 2010;49:2058–2062. Leow D, Lin S, Chittimalla SK, Fu X, Tan C-H. Angew. Chem. 2008;120:5723–5727. Angew. Chem. Int. Ed. 2008;47:5641–5645. Chan W-W, Yeung S-H, Zhou Z, Chan ASC, Yu W-Y. Org. Lett. 2010;12:604–607. Wong FM, Wang J, Hengge AC, Wu W. Org. Lett. 2007;9:1663–1665. Edwards DR, Montoya-Peleaz P, Crudden CM. Org. Lett. 2007;9:5481–5484. Stowers KJ, Sanford MS. Org. Lett. 2009;11:4584–4587. Sun K, Sachwani R, Richert KJ, Driver TG. Org. Lett. 2009;11:3598–3601.
-
-
-
Recent examples: Mugridge JS, Bergman RG, Raymond KN. Angew. Chem. 2010;122:3717–3719. Angew. Chem. Int. Ed. 2010;49:3635–3637. Penoni A, Palmisano G, Zhao Y-L, Houk KN, Volkman J, Nicholas KM. J. Am. Chem. Soc. 2009;131:653–661. Luo M, Schramm VL. J. Am. Chem. Soc. 2008;130:11617–11619. Spies MA, Toney MD. J. Am. Chem. Soc. 2007;129:10678–10685. Bailey BC, Fan H, Huffman JC, Baik M-H, Mindiola DJ. J. Am. Chem. Soc. 2007;129:8781–8793. Seo SY, Marks TJ. Chem. Eur. J. 2010;16:5148–5162. Lu Y, Qu F, Zhao Y, Small AMJ, Bradshaw J, Moore B. J. Org. Chem. 2009;74:6503–6510. Brinker UH, Lin G, Xu L, Smith WB, Mieusset J-L. J. Org. Chem. 2007;72:8434–8451.
-
-
-
Recent examples: Schneider N, Finger M, Haferkemper C, Bellemin-Laponnaz S, Hofmann P, Gade LH. Angew. Chem. 2009;121:1637–1641. Angew. Chem. Int. Ed. 2009;48:1609–1613. McCann JAB, Berti PJ. J. Am. Chem. Soc. 2008;130:5789–5797. McCann JAB, Berti PJ. J. Am. Chem. Soc. 2007;129:7055–7064. Zhang Y, Luo M, Schramm VL. J. Am. Chem. Soc. 2009;131:4685–4694. Hirschi JS, Takeya T, Hang C, Singleton DA. J. Am. Chem. Soc. 2009;131:2397–2403. Kelly KK, Hirschi JS, Singleton DA. J. Am. Chem. Soc. 2009;131:8382–8383. Oyola Y, Singleton DA. J. Am. Chem. Soc. 2009;131:3130–3131. Thomas JB, Waas JR, Harmata M, Singleton DA. J. Am. Chem. Soc. 2008;130:14544–14555. Christian CF, Takeya T, Szymanski MJ, Singleton DA. J. Org. Chem. 2007;72:6183–6189.
-
-
- Melander L, Saunders WH., Jr . Reaction Rates of Isotopic Molecules. Malabar: Krieger Publishing; 1987.
-
-
Recent applications of primary KIEs to tunneling phenomena: Panay AJ, Fitzpatrick PF. J. Am. Chem. Soc. 2010;132:5584–5585. Yoon M, Song H, Håkansson K, Marsh ENG. Biochemistry. 2010;49:3168–3173. Zhang X, Datta A, Hrovat DA, Borden WT. J. Am. Chem. Soc. 2009;131:16002–16003. Wong K-Y, Richard JP, Gao J. J. Am. Chem. Soc. 2009;131:13963–13971. Tsang W-Y, Richard JP. J. Am. Chem. Soc. 2009;131:13952–13962. Wu A, Mader EA, Datta A, Hrovat DA, Borden WT, Mayer JM. J. Am. Chem. Soc. 2009;131:11985–11997. Sharma SC, Klinman JP. J. Am. Chem. Soc. 2008;130:17632–17633.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

