Infrared and EPR spectroscopic characterization of a Ni(I) species formed by photolysis of a catalytically competent Ni(I)-CO intermediate in the acetyl-CoA synthase reaction
- PMID: 20669901
- PMCID: PMC2932805
- DOI: 10.1021/bi1010128
Infrared and EPR spectroscopic characterization of a Ni(I) species formed by photolysis of a catalytically competent Ni(I)-CO intermediate in the acetyl-CoA synthase reaction
Abstract
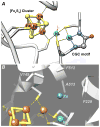
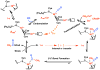
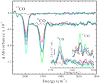
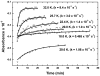
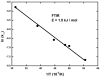
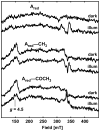
Acetyl-CoA synthase (ACS) catalyzes the synthesis of acetyl-CoA from CO, coenzyme A (CoA), and a methyl group from the CH(3)-Co(3+) site in the corrinoid iron-sulfur protein (CFeSP). These are the key steps in the Wood-Ljungdahl pathway of anaerobic CO and CO(2) fixation. The active site of ACS is the A-cluster, which is an unusual nickel-iron-sulfur cluster. There is significant evidence for the catalytic intermediacy of a CO-bound paramagnetic Ni species, with an electronic configuration of [Fe(4)S(4)](2+)-(Ni(p)(+)-CO)-(Ni(d)(2+)), where Ni(p) and Ni(d) represent the Ni centers in the A-cluster that are proximal and distal to the [Fe(4)S(4)](2+) cluster, respectively. This well-characterized Ni(p)(+)-CO intermediate is often called the NiFeC species. Photolysis of the Ni(p)(+)-CO state generates a novel Ni(p)(+) species (A(red)*) with a rhombic electron paramagnetic resonance spectrum (g values of 2.56, 2.10, and 2.01) and an extremely low (1 kJ/mol) barrier for recombination with CO. We suggest that the photolytically generated A(red)* species is (or is similar to) the Ni(p)(+) species that binds CO (to form the Ni(p)(+)-CO species) and the methyl group (to form Ni(p)-CH(3)) in the ACS catalytic mechanism. The results provide support for a binding site (an "alcove") for CO near Ni(p), indicated by X-ray crystallographic studies of the Xe-incubated enzyme. We propose that, during catalysis, a resting Ni(p)(2+) state predominates over the active Ni(p)(+) species (A(red)*) that is trapped by the coupling of a one-electron transfer step to the binding of CO, which pulls the equilibrium toward Ni(p)(+)-CO formation.
Figures


Similar articles
-
Rapid kinetic studies of acetyl-CoA synthesis: evidence supporting the catalytic intermediacy of a paramagnetic NiFeC species in the autotrophic Wood-Ljungdahl pathway.Biochemistry. 2002 Feb 12;41(6):1807-19. doi: 10.1021/bi011687i. Biochemistry. 2002. PMID: 11827525
-
Stopped-Flow Kinetics of Methyl Group Transfer between the Corrinoid-Iron-Sulfur Protein and Acetyl-Coenzyme A Synthase from Clostridium thermoaceticum.J Am Chem Soc. 2002 Jun 5;124(22):6277-84. doi: 10.1021/ja016676r. J Am Chem Soc. 2002. PMID: 12033855
-
An alcove at the acetyl-CoA synthase nickel active site is required for productive substrate CO binding and anaerobic carbon fixation.J Biol Chem. 2024 Aug;300(8):107503. doi: 10.1016/j.jbc.2024.107503. Epub 2024 Jun 27. J Biol Chem. 2024. PMID: 38944127 Free PMC article.
-
Acetyl-coenzyme A synthase: the case for a Ni(p)(0)-based mechanism of catalysis.J Biol Inorg Chem. 2004 Jul;9(5):516-24. doi: 10.1007/s00775-004-0564-x. Epub 2004 Jun 25. J Biol Inorg Chem. 2004. PMID: 15221478 Review.
-
Crystallographic evidence for a CO/CO(2) tunnel gating mechanism in the bifunctional carbon monoxide dehydrogenase/acetyl coenzyme A synthase from Moorella thermoacetica.J Biol Inorg Chem. 2004 Jul;9(5):525-32. doi: 10.1007/s00775-004-0565-9. Epub 2004 Jun 24. J Biol Inorg Chem. 2004. PMID: 15221479 Review.
Cited by
-
X-ray Absorption Spectroscopy Reveals an Organometallic Ni-C Bond in the CO-Treated Form of Acetyl-CoA Synthase.Biochemistry. 2017 Mar 7;56(9):1248-1260. doi: 10.1021/acs.biochem.6b00983. Epub 2017 Feb 23. Biochemistry. 2017. PMID: 28186407 Free PMC article.
-
Characterization of Methyl- and Acetyl-Ni Intermediates in Acetyl CoA Synthase Formed during Anaerobic CO2 and CO Fixation.J Am Chem Soc. 2023 Jun 28;145(25):13696-13708. doi: 10.1021/jacs.3c01772. Epub 2023 Jun 12. J Am Chem Soc. 2023. PMID: 37306669 Free PMC article.
-
Metal centers in the anaerobic microbial metabolism of CO and CO2.Metallomics. 2011 Aug;3(8):797-815. doi: 10.1039/c1mt00042j. Epub 2011 Jun 6. Metallomics. 2011. PMID: 21647480 Free PMC article. Review.
-
Thioester synthesis by a designed nickel enzyme models prebiotic energy conversion.Proc Natl Acad Sci U S A. 2022 Jul 26;119(30):e2123022119. doi: 10.1073/pnas.2123022119. Epub 2022 Jul 18. Proc Natl Acad Sci U S A. 2022. PMID: 35858422 Free PMC article.
-
Evidence that ferredoxin interfaces with an internal redox shuttle in Acetyl-CoA synthase during reductive activation and catalysis.Biochemistry. 2011 Jan 18;50(2):276-86. doi: 10.1021/bi101511r. Epub 2010 Dec 21. Biochemistry. 2011. PMID: 21141812 Free PMC article.
References
-
- Ragsdale SW. Life with carbon monoxide. CRC Crit Rev Biochem Mol Biol. 2004;39:165–195. - PubMed
-
- Müller V, Imkamp F, Andreas Rauwolf Küsel K, Drake HL. Molecular and Cellular Biology of Acetogenic Bacteria. In: Nakano MM, Zuber P, editors. Strict and Facultative Anaerobes: Medical and Environmental Aspects. Horizon Bioscience; Wymondham, UK: 2004. pp. 251–281.
-
- Doukov TI, Iverson TM, Seravalli J, Ragsdale SW, Drennan CL. A Ni-Fe-Cu center in a bifunctional carbon monoxide dehydrogenase/acetyl-CoA synthase. Science. 2002;298:567–572. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

