Total synthesis and evaluation of phostriecin and key structural analogues
- PMID: 20669916
- PMCID: PMC2978778
- DOI: 10.1021/jo1010203
Total synthesis and evaluation of phostriecin and key structural analogues
Abstract
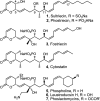
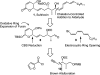
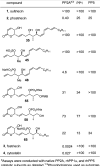
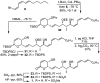
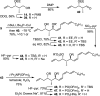
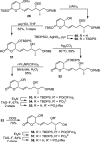
Full details of the total synthesis of phostriecin (2), the assignment of its relative and absolute stereochemistry, and the resultant structural reassignment of the natural product previously represented as sultriecin (1), a phosphate versus sulfate monoester, are detailed. Studies with authentic material confirmed that phostriecin, but not sultriecin, is an effective and selective inhibitor of protein phosphatase 2A (PP2A) defining a mechanism of action responsible for its antitumor activity. The extension of the studies to the synthesis and evaluation of a series of key synthetic analogues is disclosed that highlights the importance of the natural product phosphate monoester (vs sulfate or free alcohol, both inactive and >250-fold), the α,β-unsaturated lactone (12-fold), and the hydrophobic Z,Z,E-triene tail (C12-C22, ca. 200-fold) including the unique importance of its unsaturation (50-fold, and no longer PP2A selective).
Figures







Similar articles
-
Total synthesis, assignment of the relative and absolute stereochemistry, and structural reassignment of phostriecin (aka Sultriecin).J Am Chem Soc. 2010 Feb 24;132(7):2157-9. doi: 10.1021/ja9097252. J Am Chem Soc. 2010. PMID: 20108904 Free PMC article.
-
Total synthesis and evaluation of cytostatin, its C10-C11 diastereomers, and additional key analogues: impact on PP2A inhibition.J Am Chem Soc. 2006 Dec 27;128(51):16720-32. doi: 10.1021/ja066477d. J Am Chem Soc. 2006. PMID: 17177422 Free PMC article.
-
Total synthesis and biological evaluation of the protein phosphatase 2A inhibitor cytostatin and analogues.Chemistry. 2004 Jun 7;10(11):2759-80. doi: 10.1002/chem.200305543. Chemistry. 2004. PMID: 15195307
-
Fostriecin: chemistry and biology.Curr Med Chem. 2002 Nov;9(22):2005-32. doi: 10.2174/0929867023368809. Curr Med Chem. 2002. PMID: 12369868 Review.
-
Calyculins and related marine natural products as serine-threonine protein phosphatase PP1 and PP2A inhibitors and total syntheses of calyculin A, B, and C.Mar Drugs. 2010 Jan 21;8(1):122-72. doi: 10.3390/md80100122. Mar Drugs. 2010. PMID: 20161975 Free PMC article. Review.
Cited by
-
Catalytic Z-selective cross-metathesis in complex molecule synthesis: a convergent stereoselective route to disorazole C1.J Am Chem Soc. 2014 Nov 19;136(46):16136-9. doi: 10.1021/ja509973r. Epub 2014 Nov 7. J Am Chem Soc. 2014. PMID: 25379808 Free PMC article.
-
Total Synthesis and Structural Validation of Phosdiecin A via Asymmetric Alcohol-Mediated Carbonyl Reductive Coupling.J Am Chem Soc. 2019 Sep 4;141(35):13778-13782. doi: 10.1021/jacs.9b07512. Epub 2019 Aug 21. J Am Chem Soc. 2019. PMID: 31433167 Free PMC article.
-
Total synthesis of the potent androgen receptor antagonist (-)-arabilin: a strategic, biomimetic [1,7]-hydrogen shift.J Am Chem Soc. 2011 Dec 21;133(50):20149-51. doi: 10.1021/ja209459f. Epub 2011 Nov 29. J Am Chem Soc. 2011. PMID: 22085260 Free PMC article.
-
The Difference a Single Atom Can Make: Synthesis and Design at the Chemistry-Biology Interface.J Org Chem. 2017 Dec 1;82(23):11961-11980. doi: 10.1021/acs.joc.7b02088. Epub 2017 Oct 13. J Org Chem. 2017. PMID: 28945374 Free PMC article.
-
Formal Synthesis of Fostriecin via Asymmetric Alcohol-Mediated Carbonyl Allylation.Org Lett. 2025 May 2;27(17):4501-4506. doi: 10.1021/acs.orglett.5c01026. Epub 2025 Apr 10. Org Lett. 2025. PMID: 40209063 Free PMC article.
References
-
- Ohkuma H, Naruse N, Nishiyama Y, Tsuno T, Hoshino Y, Sawada Y, Konishi M, Oki T. J. Antibiot. 1992;45:1239. - PubMed
-
- Lewy DS, Gauss C-M, Soenen DR, Boger DL. Curr. Med. Chem. 2002;9:2005. - PubMed
-
-
Isolation: Tunac JB, Graham BD, Dobson WE. J. Antibiot. 1983;36:1595. Stampwala SS, Bunge RH, Hurley TR, Willmer NE, Brankiewicz AJ, Steinman CE, Smitka TA, French JC. J. Antibiot. 1983;36:1601. Total syntheses: Boger DL, Ichikawa S, Zhong W. J. Am. Chem. Soc. 2001;123:4161. Chavez DE, Jacobsen EN. Angew. Chem. Int. Ed. 2001;40:3667. Reddy YK, Falck JR. Org. Lett. 2002;4:969. Miyashita K, Ikejiri M, Kawasaki H, Maemura S, Imanishi T. Chem. Commun. 2002:742. Miyashita K, Ikejiri M, Kawasaki H, Maemura S, Imanishi T. J. Am. Chem. Soc. 2003;125:8238. Esumi T, Okamoto N, Hatakeyama S. Chem. Commun. 2002:3042. Fujii K, Maki K, Kanai M, Shibasaki M. Org. Lett. 2003;5:733. Trost BM, Frederiksen MU, Papillon JP, Harrington PE, Shin S, Shireman BT. J. Am. Chem. Soc. 2005;127:3666. Maki K, Motoki R, Fujii K, Kanai M, Kobayashi T, Tamura S, Shibasaki M. J. Am. Chem. Soc. 2005;127:17111. Yadav JS, Prathap I, Tadi BP. Tetrahedron Lett. 2006;47:3773. Hayashi Y, Yamaguchi H, Toyoshima M, Okado K, Toyo T, Shoji M. Org. Lett. 2008;10:1405. Sarkar SM, Wanzala EN, Shibahara S, Takahashi K, Ishihara J, Hatakeyama S. Chem. Commun. 2009:5907. Robles O, McDonald FE. Org. Lett. 2009;11:5498.
-
-
- Boger DL, Hikota M, Lewis BM. J. Org. Chem. 1997;62:1748.
- Hokanson GC, French JC. J. Org. Chem. 1985;50:462.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

