Total synthesis and evaluation of phostriecin and key structural analogues
- PMID: 20669916
- PMCID: PMC2978778
- DOI: 10.1021/jo1010203
Total synthesis and evaluation of phostriecin and key structural analogues
Abstract
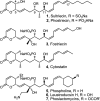
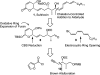
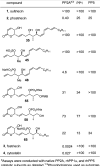
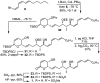
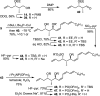
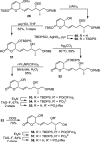
Full details of the total synthesis of phostriecin (2), the assignment of its relative and absolute stereochemistry, and the resultant structural reassignment of the natural product previously represented as sultriecin (1), a phosphate versus sulfate monoester, are detailed. Studies with authentic material confirmed that phostriecin, but not sultriecin, is an effective and selective inhibitor of protein phosphatase 2A (PP2A) defining a mechanism of action responsible for its antitumor activity. The extension of the studies to the synthesis and evaluation of a series of key synthetic analogues is disclosed that highlights the importance of the natural product phosphate monoester (vs sulfate or free alcohol, both inactive and >250-fold), the α,β-unsaturated lactone (12-fold), and the hydrophobic Z,Z,E-triene tail (C12-C22, ca. 200-fold) including the unique importance of its unsaturation (50-fold, and no longer PP2A selective).
Figures







References
-
- Ohkuma H, Naruse N, Nishiyama Y, Tsuno T, Hoshino Y, Sawada Y, Konishi M, Oki T. J. Antibiot. 1992;45:1239. - PubMed
-
- Lewy DS, Gauss C-M, Soenen DR, Boger DL. Curr. Med. Chem. 2002;9:2005. - PubMed
-
-
Isolation: Tunac JB, Graham BD, Dobson WE. J. Antibiot. 1983;36:1595. Stampwala SS, Bunge RH, Hurley TR, Willmer NE, Brankiewicz AJ, Steinman CE, Smitka TA, French JC. J. Antibiot. 1983;36:1601. Total syntheses: Boger DL, Ichikawa S, Zhong W. J. Am. Chem. Soc. 2001;123:4161. Chavez DE, Jacobsen EN. Angew. Chem. Int. Ed. 2001;40:3667. Reddy YK, Falck JR. Org. Lett. 2002;4:969. Miyashita K, Ikejiri M, Kawasaki H, Maemura S, Imanishi T. Chem. Commun. 2002:742. Miyashita K, Ikejiri M, Kawasaki H, Maemura S, Imanishi T. J. Am. Chem. Soc. 2003;125:8238. Esumi T, Okamoto N, Hatakeyama S. Chem. Commun. 2002:3042. Fujii K, Maki K, Kanai M, Shibasaki M. Org. Lett. 2003;5:733. Trost BM, Frederiksen MU, Papillon JP, Harrington PE, Shin S, Shireman BT. J. Am. Chem. Soc. 2005;127:3666. Maki K, Motoki R, Fujii K, Kanai M, Kobayashi T, Tamura S, Shibasaki M. J. Am. Chem. Soc. 2005;127:17111. Yadav JS, Prathap I, Tadi BP. Tetrahedron Lett. 2006;47:3773. Hayashi Y, Yamaguchi H, Toyoshima M, Okado K, Toyo T, Shoji M. Org. Lett. 2008;10:1405. Sarkar SM, Wanzala EN, Shibahara S, Takahashi K, Ishihara J, Hatakeyama S. Chem. Commun. 2009:5907. Robles O, McDonald FE. Org. Lett. 2009;11:5498.
-
-
- Boger DL, Hikota M, Lewis BM. J. Org. Chem. 1997;62:1748.
- Hokanson GC, French JC. J. Org. Chem. 1985;50:462.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

