Enantioselective formal synthesis of (-)-englerin A via a Rh-catalyzed [4 + 3] cycloaddition reaction
- PMID: 20669919
- PMCID: PMC2927118
- DOI: 10.1021/ol1015652
Enantioselective formal synthesis of (-)-englerin A via a Rh-catalyzed [4 + 3] cycloaddition reaction
Abstract
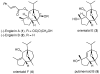
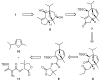
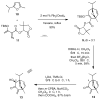
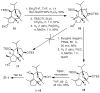
An enantioselective formal synthesis of (-)-englerin A (1) is reported. Key to the strategy is a Rh-catalyzed [4 + 3] cycloaddition reaction between furan 10 and diazo ester 11 that, following an intramolecular aldol condensation, produces the tricyclic scaffold of englerin. This strategy also provides a rapid, efficient, and stereoselective access to the biologically significant core motif of the guaiane sesquiterpenes.
Figures
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics 2009. CA Cancer J Clin. 2009;59:225–249. - PubMed
-
- Peng GP, Tian G, Huang XF, Lou FC. Phytochemistry. 2003;63:877–881. - PubMed
-
- Huang SX, Yang J, Xiao WL, Zhu YL, Li RT, Li LM, Pu JX, Li X, Li SH, Sun HD. Helv Chim Acta. 2006;89:1169–1175.
-
- Willot M, Radtke L, Könning D, Fröhlich R, Gessner VH, Strohmann C, Christmann M. Angew Chem Int Ed. 2009;48:9105–9108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources