Insights into the nitric oxide reductase mechanism of flavodiiron proteins from a flavin-free enzyme
- PMID: 20669924
- PMCID: PMC2923256
- DOI: 10.1021/bi100788y
Insights into the nitric oxide reductase mechanism of flavodiiron proteins from a flavin-free enzyme
Abstract
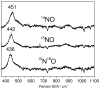
Flavodiiron proteins (FDPs) catalyze reductive scavenging of dioxygen and nitric oxide in air-sensitive microorganisms. FDPs contain a distinctive non-heme diiron/flavin mononucleotide (FMN) active site. Alternative mechanisms for the nitric oxide reductase (NOR) activity consisting of either protonation of a diiron-bridging hyponitrite or "super-reduction" of a diferrous-dinitrosyl by the proximal FMNH(2) in the rate-determining step have been proposed. To test these alternative mechanisms, we examined a deflavinated FDP (deflavo-FDP) from Thermotoga maritima. The deflavo-FDP retains an intact diiron site but does not exhibit multiturnover NOR or O(2) reductase (O(2)R) activity. Reactions of the reduced (diferrous) deflavo-FDP with nitric oxide were examined by UV-vis absorption, EPR, resonance Raman, and FTIR spectroscopies. Anaerobic addition of nitric oxide up to one NO per diferrous deflavo-FDP results in formation of a diiron-mononitrosyl complex characterized by a broad S = (1)/(2 )EPR signal arising from antiferromagnetic coupling of an S = (3)/(2) {FeNO}(7) with an S = 2 Fe(II). Further addition of NO results in two reaction pathways, one of which produces N(2)O and the diferric site and the other of which produces a stable diiron-dinitrosyl complex. Both NO-treated and as-isolated deflavo-FDPs regain full NOR and O(2)R activities upon simple addition of FMN. The production of N(2)O upon addition of NO to the mononitrosyl deflavo-FDP supports the hyponitrite mechanism, but the concomitant formation of a stable diiron-dinitrosyl complex in the deflavo-FDP is consistent with a super-reduction pathway in the flavinated enzyme. We conclude that a diiron-mononitrosyl complex is an intermediate in the NOR catalytic cycle of FDPs.
Figures








References
-
- Gardner AM, Helmick RA, Gardner PR. Flavorubredoxin, an inducible catalyst for nitric oxide reduction and detoxification in Escherichia coli. J Biol Chem. 2002;277:8172–8177. - PubMed
-
- Gomes CM, Giuffre A, Forte E, Vicente JB, Saraiva LM, Brunori M, Teixeira M. A novel type of nitric-oxide reductase. Escherichia coli flavorubredoxin. J Biol Chem. 2002;277:25273–25276. - PubMed
-
- Silaghi-Dumitrescu R, Coulter ED, Das A, Ljungdahl LG, Jameson GN, Huynh BH, Kurtz DM., Jr A flavodiiron protein and high molecular weight rubredoxin from Moorella thermoacetica with nitric oxide reductase activity. Biochemistry. 2003;42:2806–2815. - PubMed
-
- Saraiva LM, Vicente JB, Teixeira M. The role of the flavodiiron proteins in microbial nitric oxide detoxification. Adv Microb Physiol. 2004;49:77–129. - PubMed
-
- Kurtz DMJ. Flavo-diiron enzymes: nitric oxide or dioxygen reductases? Dalton Trans. 2007:4115–4121.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

