NMDA receptor contributions to visual contrast coding
- PMID: 20670835
- PMCID: PMC2913150
- DOI: 10.1016/j.neuron.2010.06.020
NMDA receptor contributions to visual contrast coding
Abstract
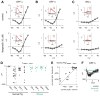
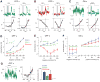
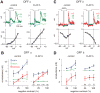
In the retina, it is not well understood how visual processing depends on AMPA- and NMDA-type glutamate receptors. Here we investigated how these receptors contribute to contrast coding in identified guinea pig ganglion cell types in vitro. NMDA-mediated responses were negligible in ON alpha cells but substantial in OFF alpha and delta cells. OFF delta cell NMDA receptors were composed of GluN2B subunits. Using a novel deconvolution method, we determined the individual contributions of AMPA, NMDA, and inhibitory currents to light responses of each cell type. OFF alpha and delta cells used NMDA receptors for encoding either the full contrast range (alpha), including near-threshold responses, or only a high range (delta). However, contrast sensitivity depended substantially on NMDA receptors only in OFF alpha cells. NMDA receptors contribute to visual contrast coding in a cell-type-specific manner. Certain cell types generate excitatory responses using primarily AMPA receptors or disinhibition.
Figures





References
-
- Berretta N, Jones RS. Tonic facilitation of glutamate release by presynaptic N-methyl-D-aspartate autoreceptors in the entorhinal cortex. Neuroscience. 1996;75:339–344. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

