Oscillations and filtering networks support flexible routing of information
- PMID: 20670837
- PMCID: PMC3125699
- DOI: 10.1016/j.neuron.2010.06.019
Oscillations and filtering networks support flexible routing of information
Abstract
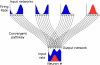
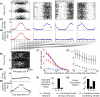
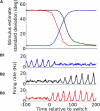
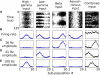
The mammalian brain exhibits profuse interregional connectivity. How information flow is rapidly and flexibly switched among connected areas remains poorly understood. Task-dependent changes in the power and interregion coherence of network oscillations suggest that such oscillations play a role in signal routing. We show that switching one of several convergent pathways from an asynchronous to an oscillatory state allows accurate selective transmission of population-coded information, which can be extracted even when other convergent pathways fire asynchronously at comparable rates. We further show that the band-pass filtering required to perform this information extraction can be implemented in a simple spiking network model with a single feed-forward interneuron layer. This constitutes a mechanism for flexible signal routing in neural circuits, which exploits sparsely synchronized network oscillations and temporal filtering by feed-forward inhibition.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

