Generation of induced pluripotent stem cells in rabbits: potential experimental models for human regenerative medicine
- PMID: 20670936
- PMCID: PMC2951210
- DOI: 10.1074/jbc.M110.150540
Generation of induced pluripotent stem cells in rabbits: potential experimental models for human regenerative medicine
Abstract
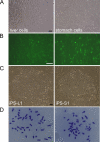
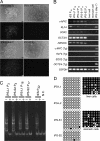
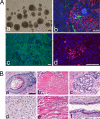
Human induced pluripotent stem (iPS) cells have the potential to establish a new field of promising regenerative medicine. Therefore, the safety and the efficiency of iPS-derived cells must be tested rigorously using appropriate animal models before human trials can commence. Here, we report the establishment of rabbit iPS cells as the first human-type iPS cells generated from a small laboratory animal species. Using lentiviral vectors, four human reprogramming genes (c-MYC, KLF4, SOX2, and OCT3/4) were introduced successfully into adult rabbit liver and stomach cells. The resulting rabbit iPS cells closely resembled human iPS cells; they formed flattened colonies with sharp edges and proliferated indefinitely in the presence of basic FGF. They expressed the endogenous pluripotency markers c-MYC, KLF4, SOX2, OCT3/4, and NANOG, whereas the introduced human genes were completely silenced. Using in vitro differentiating conditions, rabbit iPS cells readily differentiated into ectoderm, mesoderm, and endoderm. They also formed teratomas containing a variety of tissues of all three germ layers in immunodeficient mice. Thus, the rabbit iPS cells fulfilled all of the requirements for the acquisition of the fully reprogrammed state, showing high similarity to their embryonic stem cell counterparts we generated recently. However, their global gene expression analysis revealed a slight but rigid difference between these two types of rabbit pluripotent stem cells. The rabbit model should enable us to compare iPS cells and embryonic stem cells under the same standardized conditions in evaluating their ultimate feasibility for pluripotent cell-based regenerative medicine in humans.
Figures


References
-
- Martins-Taylor K., Xu R. H. (2010) J. Cell. Biochem. 109, 16–25 - PubMed
-
- Takahashi K., Yamanaka S. (2006) Cell 126, 663–676 - PubMed
-
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. (2007) Cell 131, 861–872 - PubMed
-
- Yu J., Vodyanik M. A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J. L., Tian S., Nie J., Jonsdottir G. A., Ruotti V., Stewart R., Slukvin II, Thomson J. A. (2007) Science 318, 1917–1920 - PubMed
-
- Chin M. H., Mason M. J., Xie W., Volinia S., Singer M., Peterson C., Ambartsumyan G., Aimiuwu O., Richter L., Zhang J., Khvorostov I., Ott V., Grunstein M., Lavon N., Benvenisty N., Croce C. M., Clark A. T., Baxter T., Pyle A. D., Teitell M. A., Pelegrini M., Plath K., Lowry W. E. (2009) Cell. Stem Cell 5, 111–123 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

