The Streptococcus mutans Cid and Lrg systems modulate virulence traits in response to multiple environmental signals
- PMID: 20671018
- PMCID: PMC3068699
- DOI: 10.1099/mic.0.039586-0
The Streptococcus mutans Cid and Lrg systems modulate virulence traits in response to multiple environmental signals
Abstract
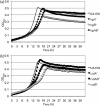
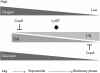
The tight control of autolysis by Streptococcus mutans is critical for proper virulence gene expression and biofilm formation. A pair of dicistronic operons, SMU.575/574 (lrgAB) and SMU.1701/1700 (designated cidAB), encode putative membrane proteins that share structural features with the bacteriophage-encoded holin family of proteins, which modulate host cell lysis during lytic infection. Analysis of S. mutans lrg and cid mutants revealed a role for these operons in autolysis, biofilm formation, glucosyltransferase expression and oxidative stress tolerance. Expression of lrgAB was repressed during early exponential phase and was induced over 1000-fold as cells entered late exponential phase, whereas cidAB expression declined from early to late exponential phase. A two-component system encoded immediately upstream of lrgAB (LytST) was required for activation of lrgAB expression, but not for cid expression. In addition to availability of oxygen, glucose levels were revealed to affect lrg and cid transcription differentially and significantly, probably through CcpA (carbon catabolite protein A). Collectively, these findings demonstrate that the Cid/Lrg system can affect several virulence traits of S. mutans, and its expression is controlled by two major environmental signals, oxygen and glucose. Moreover, cid/lrg expression is tightly regulated by LytST and CcpA.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

