Efficacy of trastuzumab in routine clinical practice and after progression for metastatic breast cancer patients: the observational Hermine study
- PMID: 20671105
- PMCID: PMC3228018
- DOI: 10.1634/theoncologist.2009-0029
Efficacy of trastuzumab in routine clinical practice and after progression for metastatic breast cancer patients: the observational Hermine study
Abstract
Background: The Hermine study observed the use of trastuzumab for metastatic breast cancer (MBC) in routine practice, including patients who received trastuzumab treatment beyond progression (TBP).
Patients and methods: The study observed 623 patients for > or = 2 years. Treatment was given according to oncologists' normal clinical practices. Endpoints included duration of treatment, efficacy, and cardiac safety. The TBP subanalysis compared overall survival (OS) in 177 patients who received first-line trastuzumab and either continued trastuzumab for > or = 30 days following progression or stopped at or before progression.
Results: The median treatment duration was 13.3 months. In the first-, second-, and third-line or beyond treatment groups, the median time to progression (TTP) were 10.3 months, 9.0 months, and 6.3 months, and the median OS times were 30.3 months, 27.1 months, and 23.2 months, respectively. Heart failure was observed in 2.6% of patients, although no cardiac-associated deaths occurred. In the TBP subanalysis, the median OS duration from treatment initiation and time of disease progression were longer in patients who continued receiving trastuzumab TBP (>27.8 months and 21.3 months, respectively) than in those who stopped (16.8 months and 4.6 months, respectively). However, the groups were not completely comparable, because patients who continued trastuzumab TBP had better prognoses at treatment initiation. The median TTP was longer in patients who continued trastuzumab TBP (10.2 months) than in those who stopped (7.1 months).
Conclusion: The Hermine findings confirm that the pivotal trials of first-line trastuzumab treatment in MBC patients are applicable in clinical practice. The subanalysis suggests that trastuzumab TBP offers a survival benefit to MBC patients treated with first-line trastuzumab.
Conflict of interest statement
The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. No financial relationships relevant to the content of this article have been disclosed by the independent peer reviewers.
Figures

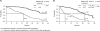
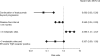
Comment in
-
Treatment beyond progression: is it moving from belief to evidence?Oncologist. 2010;15(8):796-8. doi: 10.1634/theoncologist.2010-0137. Epub 2010 Jul 29. Oncologist. 2010. PMID: 20671106 Free PMC article.
-
Trastuzumab beyond progression in retrospective analyses: an issue of equal opportunities.Oncologist. 2011;16(4):534-6. doi: 10.1634/theoncologist.2010-0329. Epub 2011 Mar 10. Oncologist. 2011. PMID: 21393343 Free PMC article.
Similar articles
-
Impact of trastuzumab treatment beyond disease progression for advanced/metastatic breast cancer on survival - results from a prospective, observational study in Germany.Breast. 2014 Oct;23(5):603-8. doi: 10.1016/j.breast.2014.06.003. Epub 2014 Jul 8. Breast. 2014. PMID: 25012046
-
Phase II Study of Weekly Paclitaxel with Trastuzumab and Pertuzumab in Patients with Human Epidermal Growth Receptor 2 Overexpressing Metastatic Breast Cancer: 5-Year Follow-up.Oncologist. 2019 Aug;24(8):e646-e652. doi: 10.1634/theoncologist.2018-0512. Epub 2019 Jan 2. Oncologist. 2019. PMID: 30602614 Free PMC article. Clinical Trial.
-
Second-Line Treatment of Her2-Positive Metastatic Breast Cancer: Trastuzumab beyond Progression or Lapatinib? A Population Based Cohort Study.PLoS One. 2015 Sep 16;10(9):e0138229. doi: 10.1371/journal.pone.0138229. eCollection 2015. PLoS One. 2015. PMID: 26375590 Free PMC article.
-
Effect of cardiac dysfunction on treatment outcomes in women receiving trastuzumab for HER2-overexpressing metastatic breast cancer.Clin Breast Cancer. 2004 Oct;5(4):293-8. doi: 10.3816/cbc.2004.n.033. Clin Breast Cancer. 2004. PMID: 15507176 Clinical Trial.
-
[A retrospective analysis of trastuzumab-based therapy in metastatic breast cancer patients at Masaryk Memorial Cancer Institute. Identification of predictive factors].Klin Onkol. 2008;21(6):348-58. Klin Onkol. 2008. PMID: 19382598 Czech.
Cited by
-
Trastuzumab Biosimilar (HLX02), Pertuzumab Plus Chemotherapy in Patients with HER2-Positive Metastatic Breast Cancer after Progression of Trastuzumab: A Prospective, Phase II Study.Cancer Res Treat. 2024 Jul;56(3):795-801. doi: 10.4143/crt.2023.1151. Epub 2023 Dec 20. Cancer Res Treat. 2024. PMID: 38147816 Free PMC article. Clinical Trial.
-
Trastuzumab beyond progression in HER2-positive advanced breast cancer: the Royal Marsden experience.Br J Cancer. 2011 May 24;104(11):1675-9. doi: 10.1038/bjc.2011.138. Epub 2011 Apr 26. Br J Cancer. 2011. PMID: 21522147 Free PMC article.
-
Real-World Data of Triplet Combination of Trastuzumab, Lapatinib, and Chemotherapy in HER2-Positive Metastatic Breast Cancer: A Multicenter Retrospective Study.Front Oncol. 2020 Mar 3;10:271. doi: 10.3389/fonc.2020.00271. eCollection 2020. Front Oncol. 2020. PMID: 32195186 Free PMC article.
-
Investigating the Correlation Between Long-Term Response in Patients with Metastatic HER2+ Breast Cancer and the Activity of Regulatory T Cells: A Retrospective Study.Breast Cancer (Dove Med Press). 2024 Sep 27;16:645-655. doi: 10.2147/BCTT.S470570. eCollection 2024. Breast Cancer (Dove Med Press). 2024. PMID: 39355199 Free PMC article.
-
New developments in the treatment of metastatic gastric cancer: focus on trastuzumab.Onco Targets Ther. 2011 Mar 24;4:21-6. doi: 10.2147/OTT.S10188. Onco Targets Ther. 2011. PMID: 21552412 Free PMC article.
References
-
- Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–2648. - PubMed
-
- Smith I, Procter M, Gelber RD, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: A randomised controlled trial. Lancet. 2007;369:29–36. - PubMed
-
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. - PubMed
-
- Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2–positive metastatic breast cancer administered as first-line treatment: The M77001 study group. J Clin Oncol. 2005;23:4265–4274. - PubMed
-
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

