The shift from a response strategy to object-in-place strategy during learning is accompanied by a matching shift in neural firing correlates in the hippocampus
- PMID: 20671146
- PMCID: PMC2920752
- DOI: 10.1101/lm.1829110
The shift from a response strategy to object-in-place strategy during learning is accompanied by a matching shift in neural firing correlates in the hippocampus
Abstract
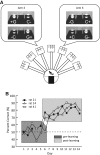
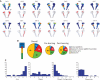
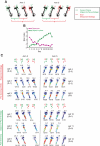
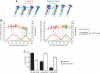
Hippocampal-dependent tasks often involve specific associations among stimuli (including egocentric information), and such tasks are therefore prone to interference from irrelevant task strategies before a correct strategy is found. Using an object-place paired-associate task, we investigated changes in neural firing patterns in the hippocampus in association with a shift in strategy during learning. We used an object-place paired-associate task in which a pair of objects was presented in two different arms of a radial maze. Each object was associated with reward only in one of the arms, thus requiring the rats to consider both object identity and its location in the maze. Hippocampal neurons recorded in CA1 displayed a dynamic transition in their firing patterns during the acquisition of the task across days, and this corresponded to a shift in strategy manifested in behavioral data. Specifically, before the rats learned the task, they chose an object that maintained a particular egocentric relationship with their body (response strategy) irrespective of the object identity. However, as the animal acquired the task, it chose an object according to both its identity and the associated location in the maze (object-in-place strategy). We report that CA1 neurons in the hippocampus changed their prospective firing correlates according to the dominant strategy (i.e., response versus object-in-place strategy) employed at a given stage of learning. The results suggest that neural firing pattern in the hippocampus is heavily influenced by the task demand hypothesized by the animal and the firing pattern changes flexibly as the perceived task demand changes.
Figures

Similar articles
-
Perirhinal cortical inactivation impairs object-in-place memory and disrupts task-dependent firing in hippocampal CA1, but not in CA3.Front Neural Circuits. 2013 Aug 14;7:134. doi: 10.3389/fncir.2013.00134. eCollection 2013. Front Neural Circuits. 2013. PMID: 23966912 Free PMC article.
-
The roles of the medial prefrontal cortex and hippocampus in a spatial paired-association task.Learn Mem. 2008 May 5;15(5):357-67. doi: 10.1101/lm.902708. Print 2008 May. Learn Mem. 2008. PMID: 18463175 Free PMC article.
-
Role of the rodent hippocampus in paired-associate learning involving associations between a stimulus and a spatial location.Behav Neurosci. 2002 Feb;116(1):63-71. doi: 10.1037//0735-7044.116.1.63. Behav Neurosci. 2002. PMID: 11895184
-
Parallel processing across neural systems: implications for a multiple memory system hypothesis.Neurobiol Learn Mem. 2004 Nov;82(3):278-98. doi: 10.1016/j.nlm.2004.07.007. Neurobiol Learn Mem. 2004. PMID: 15464410 Review.
-
Learning in a geometric model of place cell firing.Hippocampus. 2007;17(9):786-800. doi: 10.1002/hipo.20324. Hippocampus. 2007. PMID: 17598149 Review.
Cited by
-
The effects of developmental alcohol exposure on the neurobiology of spatial processing.Neurosci Biobehav Rev. 2019 Dec;107:775-794. doi: 10.1016/j.neubiorev.2019.09.018. Epub 2019 Sep 14. Neurosci Biobehav Rev. 2019. PMID: 31526818 Free PMC article. Review.
-
Hippocampus is required for paired associate memory with neither delay nor trial uniqueness.Learn Mem. 2011 Dec 14;19(1):1-8. doi: 10.1101/lm.024554.111. Print 2012 Jan. Learn Mem. 2011. PMID: 22174309 Free PMC article.
-
Still searching for the engram.Learn Behav. 2016 Sep;44(3):209-22. doi: 10.3758/s13420-016-0218-1. Learn Behav. 2016. PMID: 26944423 Free PMC article. Review.
-
Contextual behavior and neural circuits.Front Neural Circuits. 2013 May 10;7:84. doi: 10.3389/fncir.2013.00084. eCollection 2013. Front Neural Circuits. 2013. PMID: 23675321 Free PMC article. Review.
-
Rodent mnemonic similarity task performance requires the prefrontal cortex.Hippocampus. 2021 Jul;31(7):701-716. doi: 10.1002/hipo.23316. Epub 2021 Feb 19. Hippocampus. 2021. PMID: 33606338 Free PMC article.
References
-
- Burwell RD 2000. The parahippocampal region: Corticocortical connectivity. Ann N Y Acad Sci 911: 25–42 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
