Transcriptional inhibition of intestinal NHE8 expression by glucocorticoids involves Pax5
- PMID: 20671194
- PMCID: PMC2957336
- DOI: 10.1152/ajpgi.00227.2010
Transcriptional inhibition of intestinal NHE8 expression by glucocorticoids involves Pax5
Abstract
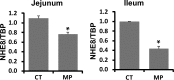
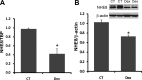
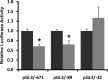
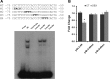
Sodium/hydrogen exchangers (NHEs) are a family of proteins that transport sodium ions into the cells by moving protons out of the cells. They play a major role in sodium absorption, cell volume regulation, and intracellular pH regulation. Three out of nine identified NHEs (NHE2, NHE3, and NHE8) are expressed on the apical membrane of intestinal epithelial cells. Glucocorticoids have been found to regulate NHE3 function in the intestine, but it is unknown if they have a similar function on NHE8 expression. Interestingly, high glucocorticoid levels in the intestine coincide chronologically with the change from high expression of NHE8 to high expression of NHE3. Studies were performed to explore the role of glucocorticoids on NHE8 expression during intestinal maturation. Brush-border membrane vesicles were isolated from intestinal epithelia, and Western blotting was performed to determine NHE8 protein expression of suckling male rats treated with methylpredisolone. Real-time PCR was used to quantitate NHE8 mRNA expression in rats and Caco-2 cells. Human NHE8 promoter activity was characterized through transfection of Caco-2 cells. Gel mobility shift assays (GMSAs) were used to identify the promoter sequences and the transcription factors involved in glucocorticoid-mediated regulation. Our results showed that the expression of NHE8 mRNA and protein was decreased in glucocorticoid-treated rats and human intestinal epithelial cells (Caco-2). The activity of the human NHE8 gene promoter transfected in Caco-2 cells was also reduced by glucocorticoid treatment. GMSAs suggested that the reduction in promoter activity in the presence of glucocorticoids was due to enhanced transcription factor Pax5 binding on the NHE8 proximal promoter region. In conclusion, this study showed that glucocorticoids inhibit NHE8 gene expression by increasing Pax5 binding on NHE8 gene promoter, suggesting an important role for Pax5 during intestinal maturation.
Figures


Similar articles
-
Transcriptional regulation of the intestinal luminal Na⁺ and Cl⁻ transporters.Biochem J. 2011 Apr 15;435(2):313-25. doi: 10.1042/BJ20102062. Biochem J. 2011. PMID: 21726200 Free PMC article. Review.
-
Epidermal growth factor inhibits intestinal NHE8 expression via reducing its basal transcription.Am J Physiol Cell Physiol. 2010 Jul;299(1):C51-7. doi: 10.1152/ajpcell.00081.2010. Epub 2010 Apr 7. Am J Physiol Cell Physiol. 2010. PMID: 20375273 Free PMC article.
-
Tumor necrosis factor-{alpha} downregulates intestinal NHE8 expression by reducing basal promoter activity.Am J Physiol Cell Physiol. 2009 Mar;296(3):C489-97. doi: 10.1152/ajpcell.00482.2008. Epub 2008 Dec 24. Am J Physiol Cell Physiol. 2009. PMID: 19109523 Free PMC article.
-
NHE2X3 DKO mice exhibit gender-specific NHE8 compensation.Am J Physiol Gastrointest Liver Physiol. 2011 Apr;300(4):G647-53. doi: 10.1152/ajpgi.00546.2010. Epub 2011 Jan 20. Am J Physiol Gastrointest Liver Physiol. 2011. PMID: 21252044 Free PMC article.
-
Functional characterization of the sodium/hydrogen exchanger 8 and its role in proliferation of colonic epithelial cells.Am J Physiol Cell Physiol. 2021 Sep 1;321(3):C471-C488. doi: 10.1152/ajpcell.00582.2020. Epub 2021 Jul 21. Am J Physiol Cell Physiol. 2021. PMID: 34288721
Cited by
-
SLC9 Gene Family: Function, Expression, and Regulation.Compr Physiol. 2018 Mar 26;8(2):555-583. doi: 10.1002/cphy.c170027. Compr Physiol. 2018. PMID: 29687889 Free PMC article. Review.
-
Transcriptional regulation of the intestinal luminal Na⁺ and Cl⁻ transporters.Biochem J. 2011 Apr 15;435(2):313-25. doi: 10.1042/BJ20102062. Biochem J. 2011. PMID: 21726200 Free PMC article. Review.
-
The Role of Plasma Membrane Sodium/Hydrogen Exchangers in Gastrointestinal Functions: Proliferation and Differentiation, Fluid/Electrolyte Transport and Barrier Integrity.Front Physiol. 2022 May 18;13:899286. doi: 10.3389/fphys.2022.899286. eCollection 2022. Front Physiol. 2022. PMID: 35665228 Free PMC article. Review.
-
NHE8 plays important roles in gastric mucosal protection.Am J Physiol Gastrointest Liver Physiol. 2013 Feb 1;304(3):G257-61. doi: 10.1152/ajpgi.00433.2012. Epub 2012 Dec 6. Am J Physiol Gastrointest Liver Physiol. 2013. PMID: 23220221 Free PMC article.
-
The Physiological Function and Potential Role of the Ubiquitous Na+/H+ Exchanger Isoform 8 (NHE8): An Overview Data.Int J Mol Sci. 2022 Sep 17;23(18):10857. doi: 10.3390/ijms231810857. Int J Mol Sci. 2022. PMID: 36142772 Free PMC article. Review.
References
-
- Adams B, Dorfler P, Aguzzi A, Kozmik Z, Urbanek P, Maurer-Fogy I, Busslinger M. Pax-5 encodes the transcription factor BSAP and is expressed in B lymphocytes, the developing CNS, and adult testis. Genes Dev 6: 1589–1607, 1992. - PubMed
-
- Bell SM, Schreiner CM, Schultheis PJ, Miller ML, Evans RL, Vorhees CV, Shull GE, Scott WJ. Targeted disruption of the murine Nhe1 locus induces ataxia, growth retardation, and seizures. Am J Physiol Cell Physiol 276: C788–C795, 1999. - PubMed
-
- Boivin GP, Schultheis PJ, Shull GE, Stemmermann GN. Variant form of diffuse corporal gastritis in NHE2 knockout mice. Comp Med 50: 511–515, 2000. - PubMed
-
- Chi N, Epstein JA. Getting your Pax straight: Pax proteins in development and disease. Trends Genet 18: 41–47, 2002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

