Transcriptional regulation of CXC-ELR chemokines KC and MIP-2 in mouse pancreatic acini
- PMID: 20671197
- PMCID: PMC2957341
- DOI: 10.1152/ajpgi.00177.2010
Transcriptional regulation of CXC-ELR chemokines KC and MIP-2 in mouse pancreatic acini
Abstract
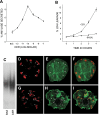
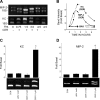
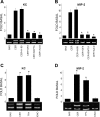
Neutrophils and their chemoattractants, the CXC-ELR chemokines keratinocyte cytokine (KC) and macrophage inflammatory protein-2 (MIP-2), play a critical role in pancreatitis. While acute pancreatitis is initiated in acinar cells, it is unclear if these are a source of CXC-ELR chemokines. KC and MIP-2 have NF-κB, activator protein-1 (AP-1) sites in their promoter regions. However, previous studies have shown increased basal and reduced caerulein-induced AP-1 activation in harvested pancreatic tissue in vitro, which limits interpreting the caerulein-induced response. Moreover, recent studies suggest that NF-κB silencing in acinar cells alone may not be sufficient to reduce inflammation in acute pancreatitis. Thus the aim of this study was to determine whether acinar cells are a source of KC and MIP-2 and to understand their transcriptional regulation. Primary overnight-cultured murine pancreatic acini were used after confirming their ability to replicate physiological and pathological acinar cell responses. Upstream signaling resulting in KC, MIP-2 upregulation was studied along with activation of the transcription factors NF-κB and AP-1. Cultured acini replicated critical responses to physiological and pathological caerulein concentrations. KC and MIP-2 mRNA levels increased in response to supramaximal but not to physiological caerulein doses. This upregulation was calcium and protein kinase C (PKC), but not cAMP, dependent. NF-κB inhibition completely prevented upregulation of KC but not MIP-2. Complete suppression of MIP-2 upregulation required dual inhibition of NF-κB and AP-1. Acinar cells are a likely source of KC and MIP-2 upregulation during pancreatitis. This upregulation is dependent on calcium and PKC. MIP-2 upregulation requires both NF-κB and AP-1 in these cells. Thus dual inhibition of NF-κB and AP-1 may be a more successful strategy to reduce inflammation in pancreatitis than targeting NF-κB alone.
Figures



Similar articles
-
IFN-gamma protects cerulein-induced acute pancreatitis by repressing NF-kappa B activation.J Immunol. 2007 Jun 1;178(11):7385-94. doi: 10.4049/jimmunol.178.11.7385. J Immunol. 2007. PMID: 17513789
-
CXCL1 regulates pulmonary host defense to Klebsiella Infection via CXCL2, CXCL5, NF-kappaB, and MAPKs.J Immunol. 2010 Nov 15;185(10):6214-25. doi: 10.4049/jimmunol.0903843. Epub 2010 Oct 11. J Immunol. 2010. PMID: 20937845 Free PMC article.
-
Substance P treatment stimulates chemokine synthesis in pancreatic acinar cells via the activation of NF-kappaB.Am J Physiol Gastrointest Liver Physiol. 2006 Dec;291(6):G1113-9. doi: 10.1152/ajpgi.00177.2006. Epub 2006 Jul 27. Am J Physiol Gastrointest Liver Physiol. 2006. PMID: 16873895
-
Role of CXCL1 in tumorigenesis of melanoma.J Leukoc Biol. 2002 Jul;72(1):9-18. J Leukoc Biol. 2002. PMID: 12101257 Free PMC article. Review.
-
Long-chain fatty acids - The turning point between 'mild' and 'severe' acute pancreatitis.Heliyon. 2024 May 22;10(11):e31296. doi: 10.1016/j.heliyon.2024.e31296. eCollection 2024 Jun 15. Heliyon. 2024. PMID: 38828311 Free PMC article. Review.
Cited by
-
Metabolically induced liver inflammation leads to NASH and differs from LPS- or IL-1β-induced chronic inflammation.Lab Invest. 2014 May;94(5):491-502. doi: 10.1038/labinvest.2014.11. Epub 2014 Feb 24. Lab Invest. 2014. PMID: 24566933
-
CXCR1/CXCR2 antagonist CXCL8(3-74)K11R/G31P blocks lung inflammation in swine barn dust-instilled mice.Pulm Pharmacol Ther. 2015 Apr;31:55-62. doi: 10.1016/j.pupt.2015.02.002. Epub 2015 Feb 12. Pulm Pharmacol Ther. 2015. PMID: 25681618 Free PMC article.
-
Changes in cytokines and chemokines in an acute pancreatitis model.Ulus Travma Acil Cerrahi Derg. 2024 Apr;30(4):229-235. doi: 10.14744/tjtes.2024.18049. Ulus Travma Acil Cerrahi Derg. 2024. PMID: 38634842 Free PMC article.
-
Macrophage inflammatory protein-2 as mediator of inflammation in acute liver injury.World J Gastroenterol. 2017 May 7;23(17):3043-3052. doi: 10.3748/wjg.v23.i17.3043. World J Gastroenterol. 2017. PMID: 28533661 Free PMC article. Review.
-
PYR-41, A Ubiquitin-Activating Enzyme E1 Inhibitor, Attenuates Lung Injury in Sepsis.Shock. 2018 Apr;49(4):442-450. doi: 10.1097/SHK.0000000000000931. Shock. 2018. PMID: 28661933 Free PMC article.
References
-
- Algul H, Tando Y, Beil M, Weber CK, Von Weyhern C, Schneider G, Adler G, Schmid RM. Different modes of NF-κB/Rel activation in pancreatic lobules. Am J Physiol Gastrointest Liver Physiol 283: G270–G281, 2002. - PubMed
-
- Andoh A, Takaya H, Saotome T, Shimada M, Hata K, Araki Y, Nakamura F, Shintani Y, Fujiyama Y, Bamba T. Cytokine regulation of chemokine (IL-8, MCP-1, and RANTES) gene expression in human pancreatic periacinar myofibroblasts. Gastroenterology 119: 211–219, 2000. - PubMed
-
- Angel P, Imagawa M, Chiu R, Stein B, Imbra RJ, Rahmsdorf HJ, Jonat C, Herrlich P, Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell 49: 729–739, 1987. - PubMed
-
- Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Ann Rev Immunol 15: 675–705, 1997. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

