Effects of add-on fluvastatin therapy in patients with chronic proteinuric nephropathy on dual renin-angiotensin system blockade: the ESPLANADE trial
- PMID: 20671225
- PMCID: PMC3001777
- DOI: 10.2215/CJN.03380410
Effects of add-on fluvastatin therapy in patients with chronic proteinuric nephropathy on dual renin-angiotensin system blockade: the ESPLANADE trial
Abstract
Background and objectives: This open, prospective, randomized trial aimed to assess the effects of statins in chronic kidney disease patients on optimized antiproteinuric treatment with combined angiotensin-converting enzyme inhibition and angiotensin receptor blockade.
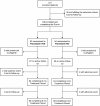
Design, setting, participants, & measurements: After 1-month benazepril therapy followed by 1-month benazepril-valsartan combined therapy (run-in), 186 consenting patients with residual proteinuria >0.5 g/24 h were randomized to 6-month benazepril-valsartan therapy alone or combined with fluvastatin. Between-groups changes in proteinuria (primary outcome), serum lipids, and GFR were compared by ANCOVA. Analyses were blinded and by intention to treat.
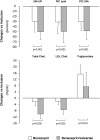
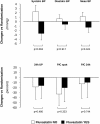
Results: During the run-in, proteinuria decreased more on benazepril-valsartan than on benazepril alone. Proteinuria reduction correlated with concomitant reduction in total, LDL, and HDL cholesterol, and apolipoprotein B and apolipoprotein A levels. After randomization, median proteinuria similarly decreased from 1.2 (0.6 to 2.2) to 1.1 (0.5 to 1.7) g/24 h on fluvastatin and from 1.5 (0.8 to 2.7) to 1.0 (0.5 to 2.4) g/24 h on benazapril-valsartan therapy alone. Fluvastatin further reduced total and LDL cholesterol and apolipoprotein B versus benazepril-valsartan alone, but did not affect serum triglycerides and GFR. Treatment was well tolerated.
Conclusions: In chronic kidney disease patients with residual proteinuria despite combined angiotensin-converting enzyme inhibitor and angiotensin receptor blockade therapy, add-on fluvastatin does not affect urinary proteins, but further reduces serum lipids and is safe. Whether combined angiotensin-converting enzyme inhibitor, angiotensin receptor blockade, and statin therapy may improve cardiovascular outcomes in this high-risk population is worth investigating.
Trial registration: ClinicalTrials.gov NCT00199927.
Figures


References
-
- Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX: Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol 17: 2034–2047, 2006 - PubMed
-
- Ruggenenti P, Perna A, Remuzzi G: Retarding progression of chronic renal disease: The neglected issue of residual proteinuria. Kidney Int 63: 2254–2261, 2003 - PubMed
-
- Vaziri ND: Dyslipidemia of chronic renal failure: The nature, mechanisms, and potential consequences. Am J Physiol Renal Physiol 290: F262–F272, 2006 - PubMed
-
- Brunskill NJ: Albumin signals the coming of age of proteinuric nephropathy. J Am Soc Nephrol 15: 504–505, 2004 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

