Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation
- PMID: 20671238
- PMCID: PMC3408082
- DOI: 10.1161/CIRCRESAHA.110.222703
Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation
Abstract
Rationale: The regenerative potential of the heart is insufficient to fully restore functioning myocardium after injury, motivating the quest for a cell-based replacement strategy. Bone marrow-derived mesenchymal stem cells (MSCs) have the capacity for cardiac repair that appears to exceed their capacity for differentiation into cardiac myocytes.
Objective: Here, we test the hypothesis that bone marrow derived MSCs stimulate the proliferation and differentiation of endogenous cardiac stem cells (CSCs) as part of their regenerative repertoire.
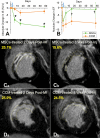
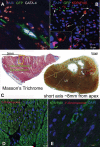
Methods and results: Female Yorkshire pigs (n=31) underwent experimental myocardial infarction (MI), and 3 days later, received transendocardial injections of allogeneic male bone marrow-derived MSCs, MSC concentrated conditioned medium (CCM), or placebo (Plasmalyte). A no-injection control group was also studied. MSCs engrafted and differentiated into cardiomyocytes and vascular structures. In addition, endogenous c-kit(+) CSCs increased 20-fold in MSC-treated animals versus controls (P<0.001), there was a 6-fold increase in GATA-4(+) CSCs in MSC versus control (P<0.001), and mitotic myocytes increased 4-fold (P=0.005). Porcine endomyocardial biopsies were harvested and plated as organotypic cultures in the presence or absence of MSC feeder layers. In vitro, MSCs stimulated c-kit(+) CSCs proliferation into enriched populations of adult cardioblasts that expressed Nkx2-5 and troponin I.
Conclusions: MSCs stimulate host CSCs, a new mechanism of action underlying successful cell-based therapeutics.
Figures





Comment in
-
Mesenchymal stem cells express c-kit.Circ Res. 2010 Nov 12;107(10):e17; author reply e18. doi: 10.1161/CIRCRESAHA.110.230961. Circ Res. 2010. PMID: 21071709 No abstract available.
References
-
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. - PubMed
-
- Boyle AJ, Schulman SP, Hare JM, Oettgen P. Is stem cell therapy ready for patients? Stem Cell Therapy for Cardiac Repair. Ready for the Next Step. Circulation. 2006;114:339–352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

