Hypoxia increases the expression of stem-cell markers and promotes clonogenicity in glioblastoma neurospheres
- PMID: 20671264
- PMCID: PMC2928980
- DOI: 10.2353/ajpath.2010.091021
Hypoxia increases the expression of stem-cell markers and promotes clonogenicity in glioblastoma neurospheres
Abstract
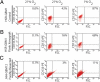
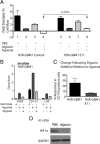
Hypoxia promotes the expansion of non-neoplastic stem and precursor cell populations in the normal brain, and is common in malignant brain tumors. We examined the effects of hypoxia on stem-like cells in glioblastoma (GBM). When GBM-derived neurosphere cultures are grown in 1% oxygen, hypoxia-inducible factor 1alpha (HIF1alpha) protein levels increase dramatically, and mRNA encoding other hypoxic response genes, such as those encoding hypoxia-inducible gene-2, lysyl oxidase, and vascular endothelial growth factor, are induced over 10-fold. Hypoxia increases the stem-like side population over fivefold, and the percentage of cells expressing CD133 threefold or more. Notch pathway ligands and targets are also induced. The rise in the stem-like fraction in GBM following hypoxia is paralleled by a twofold increase in clonogenicity. We believe HIF1alpha plays a causal role in these changes, as when oxygen-stable HIF1alpha is expressed in normoxic glioma cells CD133 is induced. We used digoxin, which has been shown to lower HIF protein levels in vitro and in vivo, to inhibit the hypoxic response. Digoxin suppressed HIF1alpha protein expression, HIF1alpha downstream targets, and slowed tumor growth in vivo. In addition, pretreatment with digoxin reduced GBM flank xenograft engraftment of hypoxic GBM cells, and daily intraperitoneal injections of digoxin were able to significantly inhibit the growth of established subcutaneous glioblastoma xenografts, and suppressed expression of vascular endothelial growth factor.
Figures




References
-
- Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. - PubMed
-
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

