CdTe Quantum Dot/Dye Hybrid System as Photosensitizer for Photodynamic Therapy
- PMID: 20671788
- PMCID: PMC2893770
- DOI: 10.1007/s11671-010-9553-x
CdTe Quantum Dot/Dye Hybrid System as Photosensitizer for Photodynamic Therapy
Abstract
We have studied the photodynamic properties of novel CdTe quantum dots-methylene blue hybrid photosensitizer. Absorption spectroscopy, photoluminescence spectroscopy, and fluorescence lifetime imaging of this system reveal efficient charge transfer between nanocrystals and the methylene blue dye. Near-infrared photoluminescence measurements provide evidence for an increased efficiency of singlet oxygen production by the methylene blue dye. In vitro studies on the growth of HepG2 and HeLa cancerous cells were also performed, they point toward an improvement in the cell kill efficiency for the methylene blue-semiconductor nanocrystals hybrid system.
Keywords: Electron transfer; Nanocrystals; Photosensitiser; Quantum dots; Singlet oxygen.
Figures
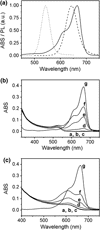
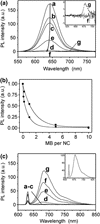
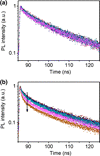

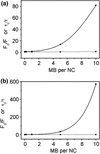
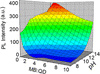

 , sample “3.3 f”) to a dilute MB solution (
, sample “3.3 f”) to a dilute MB solution ( , sample “3.3 g”), as indicated by an arrow. Bottom panel: The differential PL signal of the spectra shown in panel a. The peak singlet oxygen peak is clearly distinguishable above the noise
, sample “3.3 g”), as indicated by an arrow. Bottom panel: The differential PL signal of the spectra shown in panel a. The peak singlet oxygen peak is clearly distinguishable above the noise
References
LinkOut - more resources
Full Text Sources

