Shaping the archaeal cell envelope
- PMID: 20671907
- PMCID: PMC2910488
- DOI: 10.1155/2010/608243
Shaping the archaeal cell envelope
Abstract
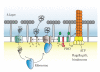
Although archaea have a similar cellular organization as other prokaryotes, the lipid composition of their membranes and their cell surface is unique. Here we discuss recent developments in our understanding of the archaeal protein secretion mechanisms, the assembly of macromolecular cell surface structures, and the release of S-layer-coated vesicles from the archaeal membrane.
Figures
Similar articles
-
Protein secretion in the Archaea: multiple paths towards a unique cell surface.Nat Rev Microbiol. 2006 Jul;4(7):537-47. doi: 10.1038/nrmicro1440. Epub 2006 Jun 6. Nat Rev Microbiol. 2006. PMID: 16755286 Review.
-
Mechanism of osmoprotection by archaeal S-layers: a theoretical study.J Struct Biol. 2007 Nov;160(2):190-9. doi: 10.1016/j.jsb.2007.08.004. Epub 2007 Aug 11. J Struct Biol. 2007. PMID: 17888677
-
The archaeal cell envelope.Nat Rev Microbiol. 2011 Jun;9(6):414-26. doi: 10.1038/nrmicro2576. Nat Rev Microbiol. 2011. PMID: 21572458 Review.
-
Archaeal Cell Walls.Subcell Biochem. 2019;92:471-493. doi: 10.1007/978-3-030-18768-2_14. Subcell Biochem. 2019. PMID: 31214995 Review.
-
Archaeal type IV pilus-like structures--evolutionarily conserved prokaryotic surface organelles.Curr Opin Microbiol. 2011 Jun;14(3):357-63. doi: 10.1016/j.mib.2011.03.002. Epub 2011 Apr 7. Curr Opin Microbiol. 2011. PMID: 21482178 Review.
Cited by
-
Molecular paleontology and complexity in the last eukaryotic common ancestor.Crit Rev Biochem Mol Biol. 2013 Jul-Aug;48(4):373-96. doi: 10.3109/10409238.2013.821444. Crit Rev Biochem Mol Biol. 2013. PMID: 23895660 Free PMC article. Review.
-
The lipopolysaccharide export pathway in Escherichia coli: structure, organization and regulated assembly of the Lpt machinery.Mar Drugs. 2014 Feb 17;12(2):1023-42. doi: 10.3390/md12021023. Mar Drugs. 2014. PMID: 24549203 Free PMC article. Review.
-
Generation of comprehensive transposon insertion mutant library for the model archaeon, Haloferax volcanii, and its use for gene discovery.BMC Biol. 2014 Dec 9;12:103. doi: 10.1186/s12915-014-0103-3. BMC Biol. 2014. PMID: 25488358 Free PMC article.
-
The Role of Secretory Pathways in Candida albicans Pathogenesis.J Fungi (Basel). 2020 Feb 24;6(1):26. doi: 10.3390/jof6010026. J Fungi (Basel). 2020. PMID: 32102426 Free PMC article. Review.
-
Potential Roles of Fungal Extracellular Vesicles during Infection.mSphere. 2016 Jun 29;1(4):e00099-16. doi: 10.1128/mSphere.00099-16. eCollection 2016 Jul-Aug. mSphere. 2016. PMID: 27390779 Free PMC article. Review.
References
-
- Koenig H. Archaeobacterial cell envelopes. Canadian Journal of Microbiology. 1988;34:395–406.
-
- Kates M. Structural analysis of phospholipids and glycolipids in extremely halophilic archaebacteria. Journal of Microbiological Methods. 1996;25(2):113–128.
-
- De Rosa M, Gambacorta A, Nicolaus B, Chappe B, Albrecht P. Isoprenoid ethers; backbone of complex lipids of the archaebacterium Sulfolobus solfataricus . Biochimica et Biophysica Acta (BBA) 1983;753(2):249–256.
-
- De Rosa M, Trincone A, Nicolaus B, Gambacorta A, di Prisco G. Life under Extreme Conditions. Berlin, Germany: Springer; 1991. Archaebacteria: lipids, membrane structures, and adaptations to environmental stresses; pp. 61–87.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

