Phosphorylation and methylation of proteasomal proteins of the haloarcheon Haloferax volcanii
- PMID: 20671954
- PMCID: PMC2910475
- DOI: 10.1155/2010/481725
Phosphorylation and methylation of proteasomal proteins of the haloarcheon Haloferax volcanii
Abstract
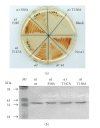
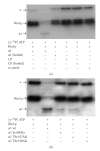
Proteasomes are composed of 20S core particles (CPs) of alpha- and beta-type subunits that associate with regulatory particle AAA ATPases such as the proteasome-activating nucleotidase (PAN) complexes of archaea. In this study, the roles and additional sites of post-translational modification of proteasomes were investigated using the archaeon Haloferax volcanii as a model. Indicative of phosphorylation, phosphatase-sensitive isoforms of alpha1 and alpha2 were detected by 2-DE immunoblot. To map these and other potential sites of post-translational modification, proteasomes were purified and analyzed by tandem mass spectrometry (MS/MS). Using this approach, several phosphosites were mapped including alpha1 Thr147, alpha2 Thr13/Ser14 and PAN-A Ser340. Multiple methylation sites were also mapped to alpha1, thus, revealing a new type of proteasomal modification. Probing the biological role of alpha1 and PAN-A phosphorylation by site-directed mutagenesis revealed dominant negative phenotypes for cell viability and/or pigmentation for alpha1 variants including Thr147Ala, Thr158Ala and Ser58Ala. An H. volcanii Rio1p Ser/Thr kinase homolog was purified and shown to catalyze autophosphorylation and phosphotransfer to alpha1. The alpha1 variants in Thr and Ser residues that displayed dominant negative phenotypes were significantly reduced in their ability to accept phosphoryl groups from Rio1p, thus, providing an important link between cell physiology and proteasomal phosphorylation.
Figures


Similar articles
-
Posttranslational modification of the 20S proteasomal proteins of the archaeon Haloferax volcanii.J Bacteriol. 2006 Nov;188(21):7521-30. doi: 10.1128/JB.00943-06. Epub 2006 Sep 1. J Bacteriol. 2006. PMID: 16950923 Free PMC article.
-
Proteasomal components required for cell growth and stress responses in the haloarchaeon Haloferax volcanii.J Bacteriol. 2008 Dec;190(24):8096-105. doi: 10.1128/JB.01180-08. Epub 2008 Oct 17. J Bacteriol. 2008. PMID: 18931121 Free PMC article.
-
Transcriptional linkage of Haloferax volcanii proteasomal genes with non-proteasomal gene neighbours including RNase P, MOSC domain and SAM-methyltransferase homologues.Microbiology (Reading). 2007 Sep;153(Pt 9):3009-3022. doi: 10.1099/mic.0.2007/008177-0. Microbiology (Reading). 2007. PMID: 17768244
-
Post-translation modification in Archaea: lessons from Haloferax volcanii and other haloarchaea.FEMS Microbiol Rev. 2013 Jul;37(4):583-606. doi: 10.1111/1574-6976.12012. Epub 2012 Dec 20. FEMS Microbiol Rev. 2013. PMID: 23167813 Free PMC article. Review.
-
Add salt, add sugar: N-glycosylation in Haloferax volcanii.Biochem Soc Trans. 2013 Feb 1;41(1):432-5. doi: 10.1042/BST20120142. Biochem Soc Trans. 2013. PMID: 23356324 Review.
Cited by
-
Ubiquitin-Like Proteasome System Represents a Eukaryotic-Like Pathway for Targeted Proteolysis in Archaea.mBio. 2016 May 17;7(3):e00379-16. doi: 10.1128/mBio.00379-16. mBio. 2016. PMID: 27190215 Free PMC article.
-
The ancient microbial RIO kinases.J Biol Chem. 2014 Apr 4;289(14):9488-92. doi: 10.1074/jbc.R113.538090. Epub 2014 Feb 19. J Biol Chem. 2014. PMID: 24554707 Free PMC article. Review.
-
Insights into the evolutionary conserved regulation of Rio ATPase activity.Nucleic Acids Res. 2018 Feb 16;46(3):1441-1456. doi: 10.1093/nar/gkx1236. Nucleic Acids Res. 2018. PMID: 29237037 Free PMC article.
-
Prokaryotic proteasomes: nanocompartments of degradation.J Mol Microbiol Biotechnol. 2013;23(4-5):321-34. doi: 10.1159/000351348. Epub 2013 Aug 5. J Mol Microbiol Biotechnol. 2013. PMID: 23920495 Free PMC article. Review.
-
Protein Ser/Thr/Tyr phosphorylation in the Archaea.J Biol Chem. 2014 Apr 4;289(14):9480-7. doi: 10.1074/jbc.R113.529412. Epub 2014 Feb 19. J Biol Chem. 2014. PMID: 24554702 Free PMC article. Review.
References
-
- Lee DH, Tanaka K, Tamura T, Chung CH, Ichihara A. PRS3 encoding an essential subunit of yeast proteasomes homologous to mammalian proteasome subunit C5. Biochemical and Biophysical Research Communications. 1992;182(2):452–460. - PubMed
-
- Lee DH, Tamura T, Chung CH, Tanaka K, Ichihara A. Molecular cloning of the yeast proteasome PRS2 gene identical to the suppressor gene scl1+ Biochemistry International. 1991;23(4):689–696. - PubMed
-
- Georgatsou E, Georgakopoulos T, Thireos G. Molecular cloning of an essential yeast gene encoding a proteasomal subunit. FEBS Letters. 1992;299(1):39–43. - PubMed
-
- Fujiwara T, Tanaka K, Orino E, et al. Proteasomes are essential for yeast proliferation. cDNA cloning and gene disruption of two major subunits. Journal of Biological Chemistry. 1990;265(27):16604–16613. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

