An 18-month study of the safety and efficacy of repeated courses of inhaled aztreonam lysine in cystic fibrosis
- PMID: 20672296
- PMCID: PMC3867945
- DOI: 10.1002/ppul.21301
An 18-month study of the safety and efficacy of repeated courses of inhaled aztreonam lysine in cystic fibrosis
Abstract
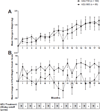
Chronic airway infection with Pseudomonas aeruginosa (PA) causes morbidity and mortality in patients with cystic fibrosis (CF). Additional anti-PA therapies are needed to improve health status and health-related quality of life. AIR-CF3 was an international 18-month, open-label study to evaluate the safety and efficacy of repeated courses of aztreonam for inhalation solution (AZLI, now marketed as Cayston®) in patients aged ≥ 6 years with CF and PA infection who previously participated in one of two Phase 3 studies: AIR-CF1 or AIR-CF2. Patients received up to nine courses (28 days on/28 days off) of 75 mg AZLI two (BID) or three times daily (TID) based on randomization in the previous trials. 274 patients, mean age 28.5 years (range: 8-74 years), participated. Mean treatment adherence was high (92.0% BID group, 88.0% TID group). Hospitalization rates were low and adverse events were consistent with CF. With each course of AZLI, FEV(1) and scores on the Cystic Fibrosis Questionnaire-Revised Respiratory Symptom scale improved and bacterial density in sputum was reduced. Benefits waned in the 28 days off therapy, but weight gain was sustained over the 18 months. There were no sustained decreases in PA susceptibility. A dose response was observed; AZLI TID-treated patients demonstrated greater improvements in lung function and respiratory symptoms over 18 months. Repeated intermittent 28-day courses of AZLI treatment were well tolerated. Clinical benefits in pulmonary function, health-related quality of life, and weight were observed with each course of therapy. AZLI is a safe and effective new therapy in patients with CF and PA airway infection.
Conflict of interest statement
Conflict of Interest Statement: Dr. Oermann received clinical research support as a site investigator conducting clinical trials for Gilead Sciences and Inspire Pharmaceuticals. Dr. Retsch-Bogart received clinical research support as a site investigator conducting clinical trial for Gilead Sciences, Inspire Pharmaceuticals, Genentech, Pathogenesis Corp., Boehringer-Ingelheim, and Cystic Fibrosis Foundation Therapeutics, Inc. Dr. Quittner was a consultant, served on an advisory board for Gilead Sciences, and has an investigator-initiated grant in another population. Dr. Gibson received clinical research support as a site investigator conducting clinical trials for Gilead Sciences, Inspire Pharmaceuticals, and Cystic Fibrosis Foundation Therapeutics. Dr. McCoy received clinical research support as a site investigator conducting clinical trials for Gilead Sciences, Inspire Pharmaceuticals, and Genentech. Dr. Montgomery is employed by Gilead Sciences. He is patent author on aztreonam for inhalation solution and Gilead Sciences is patent holder. He holds equity interest in Gilead Sciences. Dr. Cooper received clinical research support as a site investigator for clinical trials sponsored by Gilead Sciences.
Figures



References
-
- Flume PA, O’Sullivan BP, Robinson KA, Goss CH, Mogayzel PJ, Jr, Willey-Courand DB, Bujan J, Finder J, Lester M, Quittell L, Rosenblatt R, Vender RL, Hazle L, Sabadosa K, Marshall B. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176:957–969. - PubMed
-
- Gibson RL, Retsch-Bogart GZ, Oermann C, Milla C, Pilewski J, Daines C, Ahrens R, Leon K, Cohen M, McNamara S, Callahan TL, Markus R, Burns JL. Microbiology, safety, and pharmacokinetics of aztreonam lysinate for inhalation in patients with cystic fibrosis. Pediatr Pulmonol. 2006;41:656–665. - PubMed
-
- Bristol-Myers Squibb, Bristol-Myers Squibb. AZACTAM® (aztreonam for injection) [package insert] 2007
-
- Dietzsch HJ, Gottschalk B, Heyne K, Leupoid W, Wunderlich P. Cystic fibrosis: comparison of two mucolytic drugs for inhalation treatment (acetylcysteine and arginine hydrochloride) Pediatrics. 1975;55:96–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- M01 RR000400/RR/NCRR NIH HHS/United States
- M01 RR00065/RR/NCRR NIH HHS/United States
- M01 RR00039/RR/NCRR NIH HHS/United States
- M01 RR000034/RR/NCRR NIH HHS/United States
- M01 RR000082/RR/NCRR NIH HHS/United States
- M01 RR00400/RR/NCRR NIH HHS/United States
- M01 RR000065/RR/NCRR NIH HHS/United States
- M01 RR000043/RR/NCRR NIH HHS/United States
- M01 RR10710/RR/NCRR NIH HHS/United States
- M01 RR00037/RR/NCRR NIH HHS/United States
- M01 RR00070/RR/NCRR NIH HHS/United States
- M01 RR00188/RR/NCRR NIH HHS/United States
- M01 RR000069/RR/NCRR NIH HHS/United States
- M01 RR01066/RR/NCRR NIH HHS/United States
- M01 RR002172/RR/NCRR NIH HHS/United States
- M01 RR000039/RR/NCRR NIH HHS/United States
- M01 RR000188/RR/NCRR NIH HHS/United States
- M01 RR000037/RR/NCRR NIH HHS/United States
- M01 RR00827/RR/NCRR NIH HHS/United States
- M01 RR000750/RR/NCRR NIH HHS/United States
- M01 RR000489/RR/NCRR NIH HHS/United States
- M01 RR00043/RR/NCRR NIH HHS/United States
- M01 RR010710/RR/NCRR NIH HHS/United States
- M01 RR001066/RR/NCRR NIH HHS/United States
- M01 RR00750/RR/NCRR NIH HHS/United States
- M01 RR023940/RR/NCRR NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- M01 RR00069/RR/NCRR NIH HHS/United States
- M01 RR00042/RR/NCRR NIH HHS/United States
- M01 RR02172/RR/NCRR NIH HHS/United States
- M01 RR10733/RR/NCRR NIH HHS/United States
- M01 RR000042/RR/NCRR NIH HHS/United States
- M01 RR00034/RR/NCRR NIH HHS/United States
- M01 RR001070/RR/NCRR NIH HHS/United States
- M01 RR00082/RR/NCRR NIH HHS/United States
- M01 RR000070/RR/NCRR NIH HHS/United States
- M01 RR000827/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

