Three-year safety and efficacy of vicriviroc, a CCR5 antagonist, in HIV-1-infected treatment-experienced patients
- PMID: 20672447
- PMCID: PMC2917795
- DOI: 10.1097/qai.0b013e3181e2cba0
Three-year safety and efficacy of vicriviroc, a CCR5 antagonist, in HIV-1-infected treatment-experienced patients
Abstract
Background: Vicriviroc, an investigational CCR5 antagonist, demonstrated short-term safety and antiretroviral activity.
Methods: Phase 2, double-blind, randomized study of vicriviroc in treatment-experienced subjects with CCR5-using HIV-1. Vicriviroc (5, 10, or 15 mg) or placebo was added to a failing regimen with optimization of background antiretroviral medications at day 14. Subjects experiencing virologic failure and subjects completing 48 weeks were offered open-label vicriviroc.
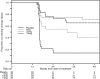
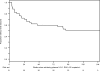
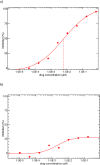
Results: One hundred eighteen subjects were randomized. Virologic failure (<1 log10 decline in HIV-1 RNA > or =16 weeks postrandomization) occurred by week 48 in 24 of 28 (86%), 12 of 30 (40%), 8 of 30 (27%), 10 of 30 (33%) of subjects randomized to placebo, 5, 10, and 15 mg, respectively. Overall, 113 subjects received vicriviroc at randomization or after virologic failure, and 52 (46%) achieved HIV-1 RNA <50 copies per milliliter within 24 weeks. Through 3 years, 49% of those achieving suppression did not experience confirmed viral rebound. Dual or mixed-tropic HIV-1 was detected in 33 (29%). Vicriviroc resistance (progressive decrease in maximal percentage inhibition on phenotypic testing) was detected in 6 subjects. Nine subjects discontinued vicriviroc due to adverse events.
Conclusions: Vicriviroc seems safe and demonstrates sustained virologic suppression through 3 years of follow-up. Further trials of vicriviroc will establish its clinical utility for the treatment of HIV-1 infection.
Trial registration: ClinicalTrials.gov NCT00082498.
Figures
References
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Nov 3, 2008. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed on December 19, 2008.
-
- Lazzarin A, Clotet B, Cooper D, et al. Efficacy of enfuvirtide in patients infected with drug-resistant HIV-1 in Europe and Australia. N Engl J Med. 2003;348:2186–95. - PubMed
-
- Lalezari JP, Henry K, O'Hearn M, et al. Enfuvirtide, an HIV-1 fusion inhibitor, for drug-resistant HIV infection in North and South America. N Engl J Med. 2003;348:2175–85. - PubMed
-
- Steigbigel RT, Cooper DA, Kumar PN, et al. BENCHMRK Study Teams Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med. 2008;359:339–54. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- AI-69432/AI/NIAID NIH HHS/United States
- AI-27661/AI/NIAID NIH HHS/United States
- M01- RR025780/RR/NCRR NIH HHS/United States
- K24-AI-51966/AI/NIAID NIH HHS/United States
- UM1 AI069556/AI/NIAID NIH HHS/United States
- AI-69495/AI/NIAID NIH HHS/United States
- U01 AI069419/AI/NIAID NIH HHS/United States
- U01 AI069502/AI/NIAID NIH HHS/United States
- AI-69418/AI/NIAID NIH HHS/United States
- UM1 AI069494/AI/NIAID NIH HHS/United States
- UM1 AI069423/AI/NIAID NIH HHS/United States
- UM1 AI069501/AI/NIAID NIH HHS/United States
- U01 AI069474/AI/NIAID NIH HHS/United States
- AI-69532/AI/NIAID NIH HHS/United States
- U01 AI069434/AI/NIAID NIH HHS/United States
- AI-69465/AI/NIAID NIH HHS/United States
- UM1 AI069472/AI/NIAID NIH HHS/United States
- U01 AI069467/AI/NIAID NIH HHS/United States
- U01 AI069423/AI/NIAID NIH HHS/United States
- AI-69556/AI/NIAID NIH HHS/United States
- M01 RR000096/RR/NCRR NIH HHS/United States
- M01 RR000046/RR/NCRR NIH HHS/United States
- AI-25859/AI/NIAID NIH HHS/United States
- AI-68634/AI/NIAID NIH HHS/United States
- U01 AI027661/AI/NIAID NIH HHS/United States
- K23-AI-55038/AI/NIAID NIH HHS/United States
- AI-69423/AI/NIAID NIH HHS/United States
- AI-34853/AI/NIAID NIH HHS/United States
- AI-69419/AI/NIAID NIH HHS/United States
- UM1 AI069424/AI/NIAID NIH HHS/United States
- AI-69439/AI/NIAID NIH HHS/United States
- U01 AI069465/AI/NIAID NIH HHS/United States
- AI-69472/AI/NIAID NIH HHS/United States
- UM1 AI069434/AI/NIAID NIH HHS/United States
- UL1 RR024996/RR/NCRR NIH HHS/United States
- UM1 AI069432/AI/NIAID NIH HHS/United States
- AI-69494/AI/NIAID NIH HHS/United States
- UL1 RR025780/RR/NCRR NIH HHS/United States
- UM1 AI069495/AI/NIAID NIH HHS/United States
- M01-RR024160/RR/NCRR NIH HHS/United States
- UM1 AI069471/AI/NIAID NIH HHS/United States
- U01 AI069439/AI/NIAID NIH HHS/United States
- U01 AI069556/AI/NIAID NIH HHS/United States
- M01-RR00096/RR/NCRR NIH HHS/United States
- U01 AI069418/AI/NIAID NIH HHS/United States
- K23 AI055038/AI/NIAID NIH HHS/United States
- AI-69467/AI/NIAID NIH HHS/United States
- AI-69434/AI/NIAID NIH HHS/United States
- M01 RR005280/RR/NCRR NIH HHS/United States
- U01 AI069532/AI/NIAID NIH HHS/United States
- K24 AI051966/AI/NIAID NIH HHS/United States
- UM1 AI069439/AI/NIAID NIH HHS/United States
- AI-32782/AI/NIAID NIH HHS/United States
- AI-69424/AI/NIAID NIH HHS/United States
- AI-69501/AI/NIAID NIH HHS/United States
- P30 AI045008/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- P30-AI-50410/AI/NIAID NIH HHS/United States
- U01 AI069501/AI/NIAID NIH HHS/United States
- AI-69474/AI/NIAID NIH HHS/United States
- U01 AI069432/AI/NIAID NIH HHS/United States
- AI-69471/AI/NIAID NIH HHS/United States
- U01 AI069450/AI/NIAID NIH HHS/United States
- U01 AI046370/AI/NIAID NIH HHS/United States
- M01-RR00052/RR/NCRR NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- AI-69450/AI/NIAID NIH HHS/United States
- UM1 AI069474/AI/NIAID NIH HHS/United States
- M01-RR00046/RR/NCRR NIH HHS/United States
- AI- 69511/AI/NIAID NIH HHS/United States
- M01 RR000052/RR/NCRR NIH HHS/United States
- U01 AI034853/AI/NIAID NIH HHS/United States
- UL1 RR024160/RR/NCRR NIH HHS/United States
- U01 AI069495/AI/NIAID NIH HHS/United States
- U01 AI069511/AI/NIAID NIH HHS/United States
- AI-46370/AI/NIAID NIH HHS/United States
- RR024979/RR/NCRR NIH HHS/United States
- UL1-RR024996/RR/NCRR NIH HHS/United States
- UM1 AI069502/AI/NIAID NIH HHS/United States
- U01 AI025859/AI/NIAID NIH HHS/United States
- UM1 AI069450/AI/NIAID NIH HHS/United States
- P30-AI-45008/AI/NIAID NIH HHS/United States
- UM1 AI069532/AI/NIAID NIH HHS/United States
- UM1 AI069465/AI/NIAID NIH HHS/United States
- U01 AI032782/AI/NIAID NIH HHS/United States
- U01-AI-68636/AI/NIAID NIH HHS/United States
- U01 AI069424/AI/NIAID NIH HHS/United States
- UM1 AI069419/AI/NIAID NIH HHS/United States
- AI-69502/AI/NIAID NIH HHS/United States
- U01 AI069494/AI/NIAID NIH HHS/United States
- UM1 AI069467/AI/NIAID NIH HHS/United States
- U01 AI069471/AI/NIAID NIH HHS/United States
- U01 AI069472/AI/NIAID NIH HHS/United States
- U01 AI068634/AI/NIAID NIH HHS/United States
- UL1 RR024979/RR/NCRR NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- UM1 AI069418/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

