Cockayne syndrome B protects against methamphetamine-enhanced oxidative DNA damage in murine fetal brain and postnatal neurodevelopmental deficits
- PMID: 20673160
- PMCID: PMC3116650
- DOI: 10.1089/ars.2009.2946
Cockayne syndrome B protects against methamphetamine-enhanced oxidative DNA damage in murine fetal brain and postnatal neurodevelopmental deficits
Abstract
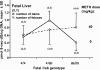
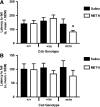
Methamphetamine (METH) increases the oxidative DNA lesion 8-oxoguanine (8-oxoG) in fetal mouse brain, and causes postnatal motor coordination deficits after in utero exposure. Like oxoguanine glycosylase 1 (OGG1), the Cockayne syndrome B (CSB) protein is involved in the repair of oxidatively damaged DNA, although its function is unclear. Here we used CSB-deficient Csb(m/m) knockout mice to investigate the developmental role of DNA oxidation and CSB in METH-initiated neurodevelopmental deficits. METH (40 mg/kg intraperitoneally) administration to pregnant Csb females on gestational day 17 increased 8-oxoG levels in Csb(m/m) fetal brains (p < 0.05). CSB modulated 8-oxoG levels independent of OGG1 activity, as 8-oxoG incision activity in fetal nuclear extracts was identical in Csb(m/m) and Csb(+/+)mice. This CSB effect was evident despite 7.1-fold higher OGG1 activity in Csb(+/+) mice compared to outbred CD-1 mice. Female Csb(m/m) offspring exposed in utero to METH exhibited motor coordination deficits postnatally (p < 0.05). In utero METH exposure did not cause dopaminergic nerve terminal degeneration, in contrast to adult exposures. This is the first evidence that CSB protects the fetus from xenobiotic-enhanced DNA oxidation and postnatal functional deficits, suggesting that oxidatively damaged DNA is developmentally pathogenic, and that fetal CSB activity may modulate the risk of reactive oxygen species-mediated adverse developmental outcomes.
Figures





Similar articles
-
Oxoguanine glycosylase 1 protects against methamphetamine-enhanced fetal brain oxidative DNA damage and neurodevelopmental deficits.J Neurosci. 2008 Sep 3;28(36):9047-54. doi: 10.1523/JNEUROSCI.2557-08.2008. J Neurosci. 2008. PMID: 18768699 Free PMC article.
-
Methamphetamine-enhanced embryonic oxidative DNA damage and neurodevelopmental deficits.Free Radic Biol Med. 2005 Aug 1;39(3):317-26. doi: 10.1016/j.freeradbiomed.2005.03.015. Epub 2005 Apr 5. Free Radic Biol Med. 2005. PMID: 15993330
-
Developmental role of nuclear factor E2-related factor 2 in mitigating methamphetamine fetal toxicity and postnatal neurodevelopmental deficits.Free Radic Biol Med. 2013 Dec;65:620-631. doi: 10.1016/j.freeradbiomed.2013.07.043. Epub 2013 Aug 7. Free Radic Biol Med. 2013. PMID: 23932974
-
Fetal oxidative stress mechanisms of neurodevelopmental deficits and exacerbation by ethanol and methamphetamine.Birth Defects Res C Embryo Today. 2016 Jun;108(2):108-30. doi: 10.1002/bdrc.21134. Birth Defects Res C Embryo Today. 2016. PMID: 27345013 Review.
-
Repair of 8-oxo-7,8-dihydroguanine in prokaryotic and eukaryotic cells: Properties and biological roles of the Fpg and OGG1 DNA N-glycosylases.Free Radic Biol Med. 2017 Jun;107:179-201. doi: 10.1016/j.freeradbiomed.2016.11.042. Epub 2016 Nov 27. Free Radic Biol Med. 2017. PMID: 27903453 Review.
Cited by
-
Breast cancer 1 (BRCA1)-deficient embryos develop normally but are more susceptible to ethanol-initiated DNA damage and embryopathies.Redox Biol. 2016 Apr;7:30-38. doi: 10.1016/j.redox.2015.11.005. Epub 2015 Nov 18. Redox Biol. 2016. PMID: 26629949 Free PMC article.
-
Daily, limited access to methamphetamine self-administration during pregnancy leads to increased methamphetamine sensitivity in adult offspring.Dev Psychobiol. 2023 Jan;65(1):e22350. doi: 10.1002/dev.22350. Dev Psychobiol. 2023. PMID: 36567658 Free PMC article.
-
De novo transcriptome assembly of the lobster cockroach Nauphoeta cinerea (Blaberidae).Genet Mol Biol. 2018 Jul/Sept.;41(3):713-721. doi: 10.1590/1678-4685-GMB-2017-0264. Epub 2018 Jul 16. Genet Mol Biol. 2018. PMID: 30043835 Free PMC article.
-
The Adverse Effects of Prenatal METH Exposure on the Offspring: A Review.Front Pharmacol. 2021 Jul 14;12:715176. doi: 10.3389/fphar.2021.715176. eCollection 2021. Front Pharmacol. 2021. PMID: 34335277 Free PMC article. Review.
References
-
- Agrawal HC. Glisson SN. Himwich WA. Developmental changes in monoamines of mouse brain. Int J Neuropharmacol. 1968;7:97–101. - PubMed
-
- Alhava E. Amphetamine toxicity in adult and developing mice. Acta Pharmacol Toxicol (Copenh) 1972;31:387–400. - PubMed
-
- Bowyer JF. Neuronal degeneration in the limbic system of weanling rats exposed to saline, hyperthermia or d-amphetamine. Brain Res. 2000;885:166–171. - PubMed
-
- Bowyer JF. Davies DL. Schmued L. Broening HW. Newport GD. Slikker W., Jr. Holson RR. Further studies of the role of hyperthermia in methamphetamine neurotoxicity. J Pharmacol Exp Ther. 1994;268:1571–1580. - PubMed
-
- Brooks PJ. DNA repair in neural cells: basic science and clinical implications. Mutat Res. 2002;509:93–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

