Innate immune stimuli modulate bone marrow-derived dendritic cell production in vitro by toll-like receptor-dependent and -independent mechanisms
- PMID: 20673241
- PMCID: PMC2999802
- DOI: 10.1111/j.1365-2567.2010.03324.x
Innate immune stimuli modulate bone marrow-derived dendritic cell production in vitro by toll-like receptor-dependent and -independent mechanisms
Abstract
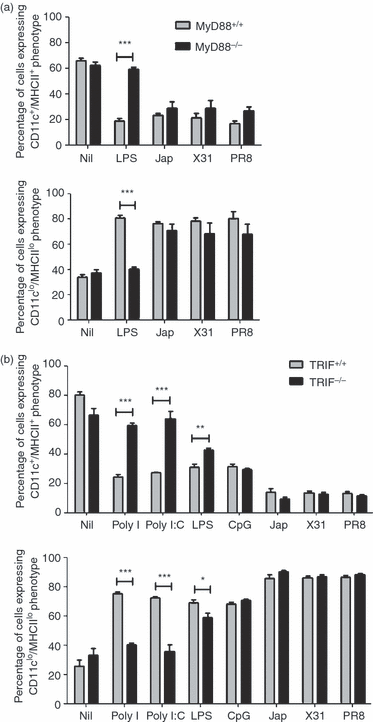
Haematopoiesis is crucial for immunity because it results in the production of leucocytes. Bacterial and viral infections alter leucocyte production by promoting granulopoiesis or lymphopoiesis. Recent studies suggest that changes in leucocyte production may be caused by the effects of inflammatory responses on the differentiation of haematopoietic progenitors in the bone marrow. We investigated the mechanisms through which infection regulates the formation of bone marrow-derived dendritic cells (BMDCs) in vitro. We mimicked infection by stimulating developing cells with molecules associated with bacteria and viruses and with inactivated influenza viruses. We showed that toll-like receptor (TLR) ligands act as modulators of haematopoiesis, and that signalling through different TLRs results in differing effects on the production of BMDCs. We demonstrated that ligands for TLR3 and influenza viruses reduce the production of BMDCs, resulting in increased neutrophil numbers, and that ligands for TLR4 and TLR9 drive the production of plasmacytoid dendritic cells. Furthermore, there are distinct signalling mechanisms involved in these effects. Signalling pathways triggered by TLR4 and TLR9 involve MyD88 and are partially mediated by the cytokine tumour necrosis factor-α (TNF-α). Mechanisms activated by TLR3 were Tir-domain-containing adaptor-inducing interferon dependent. Haematopoietic modulation induced by inactivated influenza viruses was associated with the activation of an antiviral pathway mediated by type-1 interferons.
© 2010 The Authors. Immunology © 2010 Blackwell Publishing Ltd.
Figures



Similar articles
-
HDAC6 controls innate immune and autophagy responses to TLR-mediated signalling by the intracellular bacteria Listeria monocytogenes.PLoS Pathog. 2017 Dec 27;13(12):e1006799. doi: 10.1371/journal.ppat.1006799. eCollection 2017 Dec. PLoS Pathog. 2017. PMID: 29281743 Free PMC article.
-
Innate immune responses to endosymbiotic Wolbachia bacteria in Brugia malayi and Onchocerca volvulus are dependent on TLR2, TLR6, MyD88, and Mal, but not TLR4, TRIF, or TRAM.J Immunol. 2007 Jan 15;178(2):1068-76. doi: 10.4049/jimmunol.178.2.1068. J Immunol. 2007. PMID: 17202370
-
MyD88-dependent and -independent signalling via TLR3 and TLR4 are differentially modulated by Δ9-tetrahydrocannabinol and cannabidiol in human macrophages.J Neuroimmunol. 2020 Jun 15;343:577217. doi: 10.1016/j.jneuroim.2020.577217. Epub 2020 Mar 20. J Neuroimmunol. 2020. PMID: 32244040
-
Mechanisms and pathways of innate immune activation and regulation in health and cancer.Hum Vaccin Immunother. 2014;10(11):3270-85. doi: 10.4161/21645515.2014.979640. Hum Vaccin Immunother. 2014. PMID: 25625930 Free PMC article. Review.
-
Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases.Nat Rev Immunol. 2008 Aug;8(8):594-606. doi: 10.1038/nri2358. Nat Rev Immunol. 2008. PMID: 18641647 Review.
Cited by
-
The Role of Type I IFNs in Influenza: Antiviral Superheroes or Immunopathogenic Villains?J Innate Immun. 2020;12(6):437-447. doi: 10.1159/000508379. Epub 2020 Jun 19. J Innate Immun. 2020. PMID: 32564033 Free PMC article. Review.
-
Screening of Antiviral Components of Ma Huang Tang and Investigation on the Ephedra Alkaloids Efficacy on Influenza Virus Type A.Front Pharmacol. 2019 Sep 10;10:961. doi: 10.3389/fphar.2019.00961. eCollection 2019. Front Pharmacol. 2019. PMID: 31551774 Free PMC article.
-
Pathogenesis of Respiratory Viral and Fungal Coinfections.Clin Microbiol Rev. 2022 Jan 19;35(1):e0009421. doi: 10.1128/CMR.00094-21. Epub 2021 Nov 17. Clin Microbiol Rev. 2022. PMID: 34788127 Free PMC article. Review.
-
Thymosin α1 protects from CTLA-4 intestinal immunopathology.Life Sci Alliance. 2020 Aug 14;3(10):e202000662. doi: 10.26508/lsa.202000662. Print 2020 Oct. Life Sci Alliance. 2020. PMID: 32817121 Free PMC article.
-
Candida albicans induces selective development of macrophages and monocyte derived dendritic cells by a TLR2 dependent signalling.PLoS One. 2011;6(9):e24761. doi: 10.1371/journal.pone.0024761. Epub 2011 Sep 15. PLoS One. 2011. PMID: 21935459 Free PMC article.
References
-
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. - PubMed
-
- Borrow P, Hou S, Gloster S, Ashton M, Hyland L. Virus infection-associated bone marrow B cell depletion and impairment of humoral immunity to heterologous infection mediated by TNF-alpha/LT-alpha. Eur J Immunol. 2005;35:524–32. - PubMed
-
- Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway CA. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signalling pathways. Mol Cell. 1998;2:253–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

