Hijacking the T-cell communication network by the human T-cell leukemia/lymphoma virus type 1 (HTLV-1) p12 and p8 proteins
- PMID: 20673780
- PMCID: PMC2967610
- DOI: 10.1016/j.mam.2010.07.001
Hijacking the T-cell communication network by the human T-cell leukemia/lymphoma virus type 1 (HTLV-1) p12 and p8 proteins
Abstract
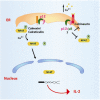
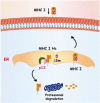
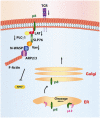
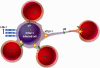
The non-structural proteins encoded by the orf-I, II, III, and IV genes of the human T-cell leukemia/lymphoma virus type 1 (HTLV-1) genome, are critical for the modulation of cellular gene expression and T-cell proliferation, the escape from cytotoxic T-cells and natural killer cells, and virus expression. In here, we review the main functions of the HTLV-1 orf-I products. The 12kDa product from orf-I (p12) is proteolytically cleaved within the endoplasmic reticulum (ER) to generate the 8kDa protein (p8). At the steady state, both proteins are expressed at similar levels in transfected T-cells. The p12 protein remains in the ER and cis-Golgi, whereas the p8 protein traffics to the cell surface and is recruited to the immunological synapse. The p12 and the p8 proteins have seemingly opposite effects on T-cells; the ER resident p12, modulates T-cell activation and proliferation, whereas p8 induces T-cell anergy. The p8 protein also increases the formation of cellular conduits, is transferred to neighboring T-cells, and increases virus transmission. The requirement for HTLV-1 infectivity of orf-I is demonstrated by the loss of virus infectivity in macaques exposed to an engineered virus, whereby expression of orf-I was ablated. Altogether the current knowledge demonstrates that the concerted activity of p8 and p12 is essential for the persistence of virus infected cells in the host.
Published by Elsevier Ltd.
Figures


References
-
- Allan JS, Leland M, Broussard S, Mone J, Hubbard G. Simian T-cell lymphotropic Viruses (STLVs) and lymphomas in African nonhuman primates. Cancer Invest. 2001;19:383–395. - PubMed
-
- Banerjee P, Feuer G, Barker E. Human T-cell leukemia virus type 1 (HTLV-1) p12I down-modulates ICAM-1 and -2 and reduces adherence of natural killer cells, thereby protecting HTLV-1-infected primary CD4+ T cells from autologous natural killer cell-mediated cytotoxicity despite the reduction of major histocompatibility complex class I molecules on infected cells. J.Virol. 2007;81:9707–9717. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

