Porous nanofibrous PLLA scaffolds for vascular tissue engineering
- PMID: 20673997
- PMCID: PMC2930107
- DOI: 10.1016/j.biomaterials.2010.07.028
Porous nanofibrous PLLA scaffolds for vascular tissue engineering
Abstract
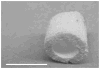
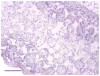
Tissue-engineered small-diameter vascular grafts are needed for patients requiring replacement of their injured coronary and below-the-knee vessels. Understanding the interactions between the scaffolds and implanted cells and therefore the phenotype control of smooth muscle cells (SMCs) is critical for constructing functional vascular grafts. In this study, the effect of nanofibrous (NF) poly-L-lactide (PLLA) scaffolds on phenotype control of human aortic smooth muscle cells (HASMCs) was investigated. A tubular NF PLLA scaffold for blood vessel regeneration was fabricated and cell seeding studies showed cell distribution throughout the scaffold. It was found that NF PLLA scaffolds preferentially supported contractile phenotype of HASMCs under the in vitro culture conditions, as evidenced by elevated gene expression level of SMCs contractile markers including smooth muscle myosin heavy chain, smoothelin and myocardin. In vivo subcutaneous implantation studies confirmed HASMCs differentiation in the implants. Taken together, the results showed promising application of the porous NF PLLA scaffolds for reconstruction of tissue-engineered vascular grafts.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures






















Similar articles
-
Heparinized PLLA/PLCL nanofibrous scaffold for potential engineering of small-diameter blood vessel: tunable elasticity and anticoagulation property.J Biomed Mater Res A. 2015 May;103(5):1784-97. doi: 10.1002/jbm.a.35315. Epub 2014 Sep 16. J Biomed Mater Res A. 2015. PMID: 25196988
-
Macroporous nanofibrous vascular scaffold with improved biodegradability and smooth muscle cells infiltration prepared by dual phase separation technique.Int J Nanomedicine. 2018 Nov 1;13:7003-7018. doi: 10.2147/IJN.S183463. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30464455 Free PMC article.
-
Engineering vascular tissue with functional smooth muscle cells derived from human iPS cells and nanofibrous scaffolds.Biomaterials. 2014 Oct;35(32):8960-9. doi: 10.1016/j.biomaterials.2014.07.011. Epub 2014 Jul 29. Biomaterials. 2014. PMID: 25085858 Free PMC article.
-
Biomimetic control of vascular smooth muscle cell morphology and phenotype for functional tissue-engineered small-diameter blood vessels.J Biomed Mater Res A. 2009 Mar 15;88(4):1104-21. doi: 10.1002/jbm.a.32318. J Biomed Mater Res A. 2009. PMID: 19097157 Review.
-
Scaffolds in tissue engineering of blood vessels.Can J Physiol Pharmacol. 2010 Sep;88(9):855-73. doi: 10.1139/y10-073. Can J Physiol Pharmacol. 2010. PMID: 20921972 Review.
Cited by
-
Patient-specific cardiovascular progenitor cells derived from integration-free induced pluripotent stem cells for vascular tissue regeneration.Biomaterials. 2015 Dec;73:51-9. doi: 10.1016/j.biomaterials.2015.09.008. Epub 2015 Sep 11. Biomaterials. 2015. PMID: 26398309 Free PMC article.
-
The Effects of Biomimetic Surface Topography on Vascular Cells: Implications for Vascular Conduits.Adv Healthc Mater. 2024 Oct;13(27):e2400335. doi: 10.1002/adhm.202400335. Epub 2024 Jul 15. Adv Healthc Mater. 2024. PMID: 38935920 Free PMC article. Review.
-
Acellular vascular grafts generated from collagen and elastin analogs.Acta Biomater. 2013 Sep;9(9):8067-74. doi: 10.1016/j.actbio.2013.05.024. Epub 2013 Jun 3. Acta Biomater. 2013. PMID: 23743129 Free PMC article.
-
Current progress in application of polymeric nanofibers to tissue engineering.Nano Converg. 2019 Nov 8;6(1):36. doi: 10.1186/s40580-019-0209-y. Nano Converg. 2019. PMID: 31701255 Free PMC article. Review.
-
Supportive features of a new hybrid scaffold for urothelium engineering.Arch Med Sci. 2015 Apr 25;11(2):438-45. doi: 10.5114/aoms.2015.50977. Epub 2015 Apr 23. Arch Med Sci. 2015. PMID: 25995764 Free PMC article.
References
-
- Kannan RY, Salacinski HJ, Butler PE, Hamilton G, Seifalian AM. Current status of prosthetic bypass grafts: a review. J Biomed Mater Res B Appl Biomater. 2005;74(1):570–581. - PubMed
-
- Nerem RM, Seliktar D. Vascular tissue engineering. Annu Rev Biomed Eng. 2001;3:225–243. - PubMed
-
- Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231(4736):397–400. - PubMed
-
- Seliktar D, Black RA, Vito RP, Nerem RM. Dynamic mechanical conditioning of collagen-gel blood vessel constructs induces remodeling in vitro. Ann Biomed Eng. 2000;28(4):351–362. - PubMed
-
- Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, et al. Functional arteries grown in vitro. Science. 1999;284(5413):489–493. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

