Nuclear localization sequence of FUS and induction of stress granules by ALS mutants
- PMID: 20674093
- PMCID: PMC2997923
- DOI: 10.1016/j.neurobiolaging.2010.06.010
Nuclear localization sequence of FUS and induction of stress granules by ALS mutants
Abstract
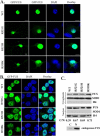
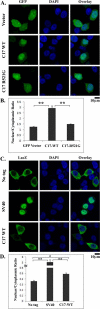
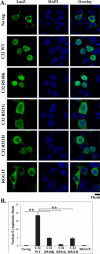
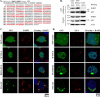
Mutations in fused in sarcoma (FUS) have been reported to cause a subset of familial amyotrophic lateral sclerosis (ALS) cases. Wild-type FUS is mostly localized in the nuclei of neurons, but the ALS mutants are partly mislocalized in the cytoplasm and can form inclusions. We demonstrate that the C-terminal 32 amino acid residues of FUS constitute an effective nuclear localization sequence (NLS) as it targeted beta-galactosidase (LacZ, 116 kDa) to the nucleus. Deletion of or the ALS mutations within the NLS caused cytoplasmic mislocalization of FUS. Moreover, we identified the poly-A binding protein (PABP1), a stress granule marker, as an interacting partner of FUS. Large PABP1-positive cytoplasmic foci (i.e. stress granules) colocalized with the mutant FUS inclusions but were absent in wild-type FUS-expressing cells. Processing bodies, which are functionally related to stress granules, were adjacent to but not colocalized with the mutant FUS inclusions. Our results suggest that the ALS mutations in FUS NLS can impair FUS nuclear localization, induce cytoplasmic inclusions and stress granules, and potentially perturb RNA metabolism.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

References
-
- Aman P, Panagopoulos I, Lassen C, Fioretos T, Mencinger M, Toresson H, Hoglund M, Forster A, Rabbitts TH, Ron D, Mandahl N, Mitelman F. Expression patterns of the human sarcoma-associated genes FUS and EWS and the genomic structure of FUS. Genomics. 1996;37(1):1–8. - PubMed
-
- Anderson P, Kedersha N. Stress granules: the Tao of RNA triage. Trends Biochem Sci. 2008;33(3):141–50. - PubMed
-
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351(3):602–11. - PubMed
-
- Baechtold H, Kuroda M, Sok J, Ron D, Lopez BS, Akhmedov AT. Human 75-kDa DNA-pairing protein is identical to the pro-oncoprotein TLS/FUS and is able to promote D-loop formation. J Biol. Chem. 1999;274(48):34337–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

