A transgenic mouse model of neuroepithelial cell specific inducible overexpression of dopamine D1-receptor
- PMID: 20674683
- PMCID: PMC2946832
- DOI: 10.1016/j.neuroscience.2010.07.036
A transgenic mouse model of neuroepithelial cell specific inducible overexpression of dopamine D1-receptor
Abstract
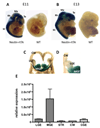
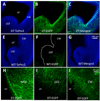
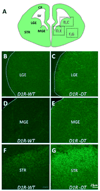
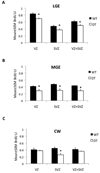
Dopamine and its receptors appear in the brain during early embryonic period suggesting a role for dopamine in brain development. In fact, dopamine receptor imbalance resulting from impaired physiological balance between D1- and D2-receptor activities can perturb brain development and lead to persisting changes in brain structure and function. Dopamine receptor imbalance can be produced experimentally using pharmacological or genetic methods. Pharmacological methods tend to activate or antagonize the receptors in all cell types. In the traditional gene knockout models the receptor imbalance occurs during development and also at maturity. Therefore, assaying the effects of dopamine imbalance on specific cell types (e.g. precursor versus postmitotic cells) or at specific periods of brain development (e.g. pre- or postnatal periods) is not feasible in these models. We describe a novel transgenic mouse model based on the tetracycline dependent inducible gene expression system in which dopamine D1-receptor transgene expression is induced selectively in neuroepithelial cells of the embryonic brain at experimenter-chosen intervals of brain development. In this model, doxycycline-induced expression of the transgene causes significant overexpression of the D1-receptor and significant reductions in the incorporation of the S-phase marker bromodeoxyuridine into neuroepithelial cells of the basal and dorsal telencephalon indicating marked effects on telencephalic neurogenesis. The D1-receptor overexpression occurs at higher levels in the medial ganglionic eminence (MGE) than the lateral ganglionic eminence (LGE) or cerebral wall (CW). Moreover, although the transgene is induced selectively in the neuroepithelium, D1-receptor protein overexpression appears to persist in postmitotic cells. The mouse model can be modified for neuroepithelial cell-specific inducible expression of other transgenes or induction of the D1-receptor transgene in other cells in specific brain regions by crossbreeding the mice with transgenic mouse lines available already.
Copyright © 2010 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Dopamine modulates cell cycle in the lateral ganglionic eminence.J Neurosci. 2003 Apr 1;23(7):2840-50. doi: 10.1523/JNEUROSCI.23-07-02840.2003. J Neurosci. 2003. PMID: 12684471 Free PMC article.
-
Locomotor behavior of dopamine D1 receptor transgenic/D2 receptor deficient hybrid mice.Brain Res. 2001 Jun 29;905(1-2):142-51. doi: 10.1016/s0006-8993(01)02522-7. Brain Res. 2001. PMID: 11423089
-
Influence of dopamine on precursor cell proliferation and differentiation in the embryonic mouse telencephalon.Dev Neurosci. 2004 Mar-Aug;26(2-4):229-44. doi: 10.1159/000082140. Dev Neurosci. 2004. PMID: 15711063 Free PMC article.
-
From neuroepithelial cells to neurons: changes in the physiological properties of neuroepithelial stem cells.Arch Biochem Biophys. 2013 Jun;534(1-2):64-70. doi: 10.1016/j.abb.2012.07.016. Epub 2012 Aug 7. Arch Biochem Biophys. 2013. PMID: 22892549 Review.
-
Homeoprotein signaling in the developing and adult nervous system.Neuron. 2015 Mar 4;85(5):911-25. doi: 10.1016/j.neuron.2015.01.019. Neuron. 2015. PMID: 25741720 Free PMC article. Review.
Cited by
-
Constitutive activity of a G protein-coupled receptor, DRD1, contributes to human cerebral organoid formation.Stem Cells. 2020 May;38(5):653-665. doi: 10.1002/stem.3156. Epub 2020 Feb 18. Stem Cells. 2020. PMID: 32052915 Free PMC article.
References
-
- Aoki Y, Huang Z, Thomas SS, Bhide PG, Huang I, Moskowitz MA, Reeves SA. Increased susceptibility to ischemia-induced brain damage in transgenic mice overexpressing a dominant negative form of SHP2. Faseb J. 2000;14:1965–1973. - PubMed
-
- Bhide PG. Cell cycle kinetics in the embryonic mouse corpus striatum. J Comp Neurol. 1996;374:506–522. - PubMed
-
- Bhide PG. Dopamine, cocaine and the development of cerebral cortical cytoarchitecture: A review of current concepts. Seminars Cell Dev Biol. 2009;20:395–402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

