Phosphatidylinositol-3-kinase p110γ contributes to bile salt-induced apoptosis in primary rat hepatocytes and human hepatoma cells
- PMID: 20675006
- PMCID: PMC2949543
- DOI: 10.1016/j.jhep.2010.05.015
Phosphatidylinositol-3-kinase p110γ contributes to bile salt-induced apoptosis in primary rat hepatocytes and human hepatoma cells
Abstract
Background & aims: Glycochenodeoxycholate (GCDC) and taurolithocholate (TLC) are hepatotoxic and cholestatic bile salts, whereas tauroursodeoxycholate (TUDC) is cytoprotective and anticholestatic. Yet they all act, in part, through phosphatidylinositol-3-kinase(PI3K)-dependent mechanisms ("PI3K-paradox"). Hepatocytes express three catalytic PI3K Class I isoforms (p110α/β/γ), specific functions of which, in liver, are unclear. In other cell types, p110γ is associated with detrimental effects. To shed light on the PI3K enigma, we determined whether hydrophobic and hydrophilic bile salts differentially activate distinct p110 isoforms in hepatocytes, and whether p110γ mediates bile salt-induced hepatocyte cell death.
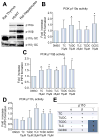
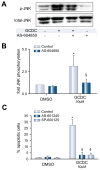
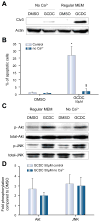
Methods: Isoform-specific PI3K activity assays were established to determine isoform activation by bile salts in rat hepatocytes. Activation of Akt and JNK was determined by immunoblotting. Following stimulation with hydrophobic bile salts, hepatocellular apoptosis was determined morphologically after Hoechst staining and by analysis of caspase-3/-7 activity or caspase-3 cleavage. Activity or expression of PI3K p110γ was inhibited pharmacologically (AS604850) or by knock-down using specific siRNA.
Results: All bile salts tested activated p110β, while p110α was activated by TUDC and GCDC. Intriguingly, only hydrophobic bile salts activated p110γ. Inhibition of p110γ attenuated GCDC-induced Akt- and JNK-activation, but did not alter TUDC- or cAMP-induced Akt-signaling in rat hepatocytes. Inhibition or knock-down of p110γ markedly attenuated hydrophobic bile salt-induced apoptosis in rat hepatocytes and human hepatoma cell lines but did not alter Fas-, tumor necrosis factor α- and etoposide-induced apoptosis. Depletion of Ca(++) prevented GCDC-induced toxicity in rat hepatocytes but did not affect GCDC-induced Akt- and JNK-activation.
Conclusions: PI3K p110γ is activated by hydrophobic, but not hydrophilic bile salts. Bile salt-induced hepatocyte apoptosis is partly mediated via a PI3K p110γ dependent signaling pathway, potentially involving JNK.
Copyright © 2010 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Figures


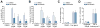

References
-
- Guicciardi ME, Gores GJ. Bile acid-mediated hepatocyte apoptosis and cholestatic liver disease. Dig Liver Dis. 2002;34:387–392. - PubMed
-
- Rust C, Bauchmuller K, Fickert P, Fuchsbichler A, Beuers U. Phosphatidylinositol 3-kinase-dependent signaling modulates taurochenodeoxycholic acid-induced liver injury and cholestasis in perfused rat livers. Am J Physiol Gastrointest Liver Physiol. 2005;289:G88–94. - PubMed
-
- Wimmer R, Hohenester S, Pusl T, Denk GU, Rust C, Beuers U. Tauroursodeoxycholic acid exerts anticholestatic effects by a cooperative cPKC alpha-/PKA-dependent mechanism in rat liver. Gut. 2008;57:1448–1454. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

