Role of peripheral endothelin receptors in an animal model of complex regional pain syndrome type 1 (CRPS-I)
- PMID: 20675053
- PMCID: PMC4474643
- DOI: 10.1016/j.pain.2010.07.003
Role of peripheral endothelin receptors in an animal model of complex regional pain syndrome type 1 (CRPS-I)
Abstract
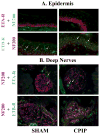
Chronic post-ischemic pain (CPIP) is an animal model of CRPS-I developed using a 3-h ischemia-reperfusion injury of the rodent hind paw. The contribution of local endothelin to nociception has been evaluated in CPIP mice by measuring sustained nociceptive behaviors (SNBs) following intraplantar injection of endothelin-1 or -2 (ET-1, ET-2). The effects of local BQ-123 (ETA-R antagonist), BQ-788 (ETB-R antagonist), IRL-1620 (ETB-R agonist) and naloxone (opioid antagonist) were assessed on ET-induced SNBs and/or mechanical and cold allodynia in CPIP mice. ETA-R and ETB-R expression was assessed using immunohistochemistry and Western blot analysis. Compared to shams, CPIP mice exhibited hypersensitivity to local ET-1 and ET-2. BQ-123 reduced ET-1- and ET-2-induced SNBs in both sham and CPIP animals, but not mechanical or cold allodynia. BQ-788 enhanced ET-1- and ET-2-induced SNBs in both sham and CPIP mice, and cold allodynia in CPIP mice. IRL-1620 displayed a non-opioid anti-nociceptive effect on ET-1- and ET-2-induced SNBs and mechanical allodynia in CPIP mice. The distribution of ETA-R and ETB-R was similar in plantar skin of sham and CPIP mice, but both receptors were over-expressed in plantar muscles of CPIP mice. This study shows that ETA-R and ETB-R have differing roles in nociception for sham and CPIP mice. CPIP mice exhibit more local endothelin-induced SNBs, develop a novel local ETB-R agonist-induced (non-opioid) analgesia, and exhibit over-expression of both receptors in plantar muscles, but not skin. The effectiveness of local ETB-R agonists as anti-allodynic treatments in CPIP mice holds promise for novel therapies in CRPS-I patients.
Copyright © 2010 International Association for the Study of Pain. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures






Similar articles
-
Antiallodynic effect through spinal endothelin-B receptor antagonism in rat models of complex regional pain syndrome.Neurosci Lett. 2015 Jan 1;584:45-9. doi: 10.1016/j.neulet.2014.10.005. Epub 2014 Oct 22. Neurosci Lett. 2015. PMID: 25451723
-
Local injection of a selective endothelin-B receptor agonist inhibits endothelin-1-induced pain-like behavior and excitation of nociceptors in a naloxone-sensitive manner.J Neurosci. 2002 Sep 1;22(17):7788-96. doi: 10.1523/JNEUROSCI.22-17-07788.2002. J Neurosci. 2002. PMID: 12196602 Free PMC article.
-
Characterization of ETB receptors mediating contractions induced by endothelin-1 or IRL 1620 in guinea-pig isolated airways: effects of BQ-123, FR139317 or PD 145065.Br J Pharmacol. 1994 Apr;111(4):1009-16. doi: 10.1111/j.1476-5381.1994.tb14844.x. Br J Pharmacol. 1994. PMID: 8032583 Free PMC article.
-
Endothelin.Pharmacol Rev. 2016 Apr;68(2):357-418. doi: 10.1124/pr.115.011833. Pharmacol Rev. 2016. PMID: 26956245 Free PMC article. Review.
-
A hypothesis for the cause of complex regional pain syndrome-type I (reflex sympathetic dystrophy): pain due to deep-tissue microvascular pathology.Pain Med. 2010 Aug;11(8):1224-38. doi: 10.1111/j.1526-4637.2010.00911.x. Pain Med. 2010. PMID: 20704671 Free PMC article. Review.
Cited by
-
Differential association of receptor-Gβγ complexes with β-arrestin2 determines recycling bias and potential for tolerance of δ opioid receptor agonists.J Neurosci. 2012 Apr 4;32(14):4827-40. doi: 10.1523/JNEUROSCI.3734-11.2012. J Neurosci. 2012. PMID: 22492038 Free PMC article.
-
Manual Therapy Reduces Pain Behavior and Oxidative Stress in a Murine Model of Complex Regional Pain Syndrome Type I.Brain Sci. 2019 Aug 10;9(8):197. doi: 10.3390/brainsci9080197. Brain Sci. 2019. PMID: 31405150 Free PMC article.
-
Crosstalk between astrocytic CXCL12 and microglial CXCR4 contributes to the development of neuropathic pain.Mol Pain. 2016 Mar 8;12:1744806916636385. doi: 10.1177/1744806916636385. Print 2016. Mol Pain. 2016. PMID: 27030717 Free PMC article.
-
New perspectives on the endothelin axis in pain.Pharmacol Res. 2011 Jun;63(6):532-40. doi: 10.1016/j.phrs.2011.02.002. Epub 2011 Feb 23. Pharmacol Res. 2011. PMID: 21352917 Free PMC article. Review. No abstract available.
-
Epigenetic signature of chronic low back pain in human T cells.Pain Rep. 2021 Nov 3;6(4):e960. doi: 10.1097/PR9.0000000000000960. eCollection 2021 Nov-Dec. Pain Rep. 2021. PMID: 34746619 Free PMC article.
References
-
- Baamonde A, Lastra A, Villazón M, Bordallo J, Hidalgo A, Menéndez L. Involvement of endogenous endothelins in thermal and mechanical inflammatory hyperalgesia in mice. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:245–51. - PubMed
-
- Berti-Mattera LN, Gariepy CE, Burke RM, Hall AK. Reduced expression of endothelin B receptors and mechanical hyperalgesia in experimental chronic diabetes. Exp Neurol. 2006;201:399–406. - PubMed
-
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. - PubMed
-
- Chichorro JG, Zampronio AR, Rae GA. Endothelin ET(B) receptor antagonist reduces mechanical allodynia in rats with trigeminal neuropathic pain. Exp Biol Med (Maywood) 2006;231:1136–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

