Critical role for lactate dehydrogenase A in aerobic glycolysis that sustains pulmonary microvascular endothelial cell proliferation
- PMID: 20675437
- PMCID: PMC2957419
- DOI: 10.1152/ajplung.00274.2009
Critical role for lactate dehydrogenase A in aerobic glycolysis that sustains pulmonary microvascular endothelial cell proliferation
Abstract
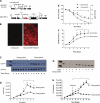
Pulmonary microvascular endothelial cells possess both highly proliferative and angiogenic capacities, yet it is unclear how these cells sustain the metabolic requirements essential for such growth. Rapidly proliferating cells rely on aerobic glycolysis to sustain growth, which is characterized by glucose consumption, glucose fermentation to lactate, and lactic acidosis, all in the presence of sufficient oxygen concentrations. Lactate dehydrogenase A converts pyruvate to lactate necessary to sustain rapid flux through glycolysis. We therefore tested the hypothesis that pulmonary microvascular endothelial cells express lactate dehydrogenase A necessary to utilize aerobic glycolysis and support their growth. Pulmonary microvascular endothelial cell (PMVEC) growth curves were conducted over a 7-day period. PMVECs consumed glucose, converted glucose into lactate, and acidified the media. Restricting extracellular glucose abolished the lactic acidosis and reduced PMVEC growth, as did replacing glucose with galactose. In contrast, slow-growing pulmonary artery endothelial cells (PAECs) minimally consumed glucose and did not develop a lactic acidosis throughout the growth curve. Oxygen consumption was twofold higher in PAECs than in PMVECs, yet total cellular ATP concentrations were twofold higher in PMVECs. Glucose transporter 1, hexokinase-2, and lactate dehydrogenase A were all upregulated in PMVECs compared with their macrovascular counterparts. Inhibiting lactate dehydrogenase A activity and expression prevented lactic acidosis and reduced PMVEC growth. Thus PMVECs utilize aerobic glycolysis to sustain their rapid growth rates, which is dependent on lactate dehydrogenase A.
Figures



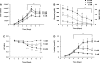


References
-
- Al-Mehdi AB. Mechanotransduction of shear-stress at the mitochondria. In: Advances in Biochemistry in Health and Disease: Mitochondria: The Dynamic Organelle, edited by Shaffer SW, Suleimon MS. New York: Springer, 2007.
-
- Alvarez DF, Huang L, King JA, ElZarrad MK, Yoder MC, Stevens T. Lung microvascular endothelium is enriched with progenitor cells that exhibit vasculogenic capacity. Am J Physiol Lung Cell Mol Physiol 294: L419– L430, 2008 - PubMed
-
- Brand KA, Hermfisse U. Aerobic glycolysis by proliferating cells: a protective strategy against reactive oxygen species. FASEB J 11: 388– 395, 1997 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

