NENA, a Lotus japonicus homolog of Sec13, is required for rhizodermal infection by arbuscular mycorrhiza fungi and rhizobia but dispensable for cortical endosymbiotic development
- PMID: 20675572
- PMCID: PMC2929109
- DOI: 10.1105/tpc.109.069807
NENA, a Lotus japonicus homolog of Sec13, is required for rhizodermal infection by arbuscular mycorrhiza fungi and rhizobia but dispensable for cortical endosymbiotic development
Abstract
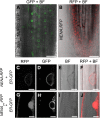
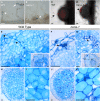
Legumes form symbioses with arbuscular mycorrhiza (AM) fungi and nitrogen fixing root nodule bacteria. Intracellular root infection by either endosymbiont is controlled by the activation of the calcium and calmodulin-dependent kinase (CCaMK), a central regulatory component of the plant's common symbiosis signaling network. We performed a microscopy screen for Lotus japonicus mutants defective in AM development and isolated a mutant, nena, that aborted fungal infection in the rhizodermis. NENA encodes a WD40 repeat protein related to the nucleoporins Sec13 and Seh1. Localization of NENA to the nuclear rim and yeast two-hybrid experiments indicated a role for NENA in a conserved subcomplex of the nuclear pore scaffold. Although nena mutants were able to form pink nodules in symbiosis with Mesorhizobium loti, root hair infection was not observed. Moreover, Nod factor induction of the symbiotic genes NIN, SbtM4, and SbtS, as well as perinuclear calcium spiking, were impaired. Detailed phenotypic analyses of nena mutants revealed a rhizobial infection mode that overcame the lack of rhizodermal responsiveness and carried the hallmarks of crack entry, including a requirement for ethylene. CCaMK-dependent processes were only abolished in the rhizodermis but not in the cortex of nena mutants. These data support the concept of tissue-specific components for the activation of CCaMK.
Figures






References
-
- Alber F., Dokudovskaya S., Veenhoff L.M., Zhang W., Kipper J., Devos D., Suprapto A., Karni-Schmidt O., Williams R., Chait B.T., Sali A., Rout M.P. (2007). The molecular architecture of the nuclear pore complex. Nature 450: 695–701 - PubMed
-
- Ané J.M., et al. (2004). Medicago truncatula DMI1 required for bacterial and fungal symbioses in legumes. Science 303: 1364–1367 - PubMed
-
- Arnold K., Bordoli L., Kopp J., Schwede T. (2006). The SWISS-MODEL workspace: A web-based environment for protein structure homology modelling. Bioinformatics 22: 195–201 - PubMed
Publication types
MeSH terms
Substances
Associated data
- GDB/P55735
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

